Physiology of Thermoregulation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effect of Ethanol on Thermoregulation in the Goldfish, Carassius Auratus
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1986 Effect of ethanol on thermoregulation in the goldfish, Carassius auratus Candace Sharon O'Connor Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Biology Commons, and the Physiology Commons Let us know how access to this document benefits ou.y Recommended Citation O'Connor, Candace Sharon, "Effect of ethanol on thermoregulation in the goldfish, Carassius auratus" (1986). Dissertations and Theses. Paper 3703. https://doi.org/10.15760/etd.5587 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. AN ABSTRACT OF THE THESIS of Candace Sharon O'Connor for the Master of Science in Biology presented May 16, 1986. Title: Effect of Ethanol on Thermoregulation in the Goldfish, Carassius auratus. APPROVED BY MEMBERS OF THE TIIBSIS COMMITTEE: Leonard Simpson In an attempt to elucidate the mechanism by which ethanol affects vertebrate thermoregulation, the effect of ethanol on temperature selection was studied in the goldfish, Carassius auratus. Ethanol was administered to 10 to 15 g fish by mixing it in the water of a temperature gradient. The dose response curve was very steep between 0.5% (v/v) ethanol (no response) and 0.7% (significant lowering of selected temperature in treated fish). Fish were exposed to concentrations of ethanol as high as 1.7%, at which concentration most experimental fish lost their ability to swim upright in the water. -

Temperature Regulation.Pdf
C H A P T E R 13 Thermal Physiology PowerPoint® Lecture Slides prepared by Stephen Gehnrich, Salisbury University Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Thermal Tolerance of Animals Eurytherm Can tolerate a wide range of ambient temperatures Stenotherm Can tolerate only a narrow range of ambient temperatures Eurytherms can occupy a greater number of thermal niches than stenotherms Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Acclimation of metabolic rate to temperature in a poikilotherm (chronic response) (5 weeks) (5 weeks) Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Compensation for temperature changes (chronic response) “Temperature acclimation” Partial compensation Full compensation Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Temperature is important for animal tissues for two reasons: 1. Temperature affects the rates of tissue processes (metabolic rates, biochemical reaction, biophysical reactions) 2. Temperature affects the molecular conformations, and therefore, the functional states of molecules. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Different species have evolved different molecular form of enzymes. All six species have about the same enzyme-substrate affinity when they are at their respective body temperature. Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings The enzyme of Antarctic fish is very -

Ostrich Production Systems Part I: a Review
11111111111,- 1SSN 0254-6019 Ostrich production systems Food and Agriculture Organization of 111160mmi the United Natiorp str. ro ucti s ct1rns Part A review by Dr M.M. ,,hanawany International Consultant Part II Case studies by Dr John Dingle FAO Visiting Scientist Food and , Agriculture Organization of the ' United , Nations Ot,i1 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. M-21 ISBN 92-5-104300-0 Reproduction of this publication for educational or other non-commercial purposes is authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Information Division, Food and Agriculture Organization of the United Nations, Viale dells Terme di Caracalla, 00100 Rome, Italy. C) FAO 1999 Contents PART I - PRODUCTION SYSTEMS INTRODUCTION Chapter 1 ORIGIN AND EVOLUTION OF THE OSTRICH 5 Classification of the ostrich in the animal kingdom 5 Geographical distribution of ratites 8 Ostrich subspecies 10 The North -

Effect of Body Temperature on the Pattern of Spontaneous Breathing in Extremely Low Birth Weight Infants Supported by Proportional Assist Ventilation
0031-3998/03/5403-0332 PEDIATRIC RESEARCH Vol. 54, No. 3, 2003 Copyright © 2003 International Pediatric Research Foundation, Inc. Printed in U.S.A. Effect of Body Temperature on the Pattern of Spontaneous Breathing in Extremely Low Birth Weight Infants Supported by Proportional Assist Ventilation ESTHER RIEGER-FACKELDEY, SUSANNE SCHALLER-BALS, AND ANDREAS SCHULZE Department of Obstetrics & Gynecology–Grosshadern, Division of Neonatology, Ludwig Maximilian University of Munich, D-81377 Munich, Germany ABSTRACT The optimum body temperature for infants Ͻ1000 g is un- 0.001) as a result of a difference in RR (8%; p Ͻ 0.001). The Ϯ known. We investigated body temperature effects on spontane- infants maintained their blood CO2 levels and Vt (5.25 0.6 ous breathing using proportional assist ventilation (PAV), be- versus 5.19 Ϯ 0.6 mL/kg). Incidence and duration of respiratory cause this mode supports spontaneous breathing such that all pauses were not different between conditions. Extremely imma- breathing pattern variables remain controlled by the infant. ture infants who are supported by PAV modify their spontaneous Minute volume (MV), respiratory rate (RR), tidal volume (Vt), breathing in response to changes in thermal environment such incidence and duration of respiratory pauses, arterial oxygen that PCO2 levels are appropriately maintained early in postnatal Ͻ desaturations 85%, and arterial PCO2 levels will remain unaf- life. This response pattern occurred consistently and is currently fected by targeting core body temperature to 36.1–36.5°C (low of uncertain clinical significance. (Pediatr Res 54: 332–336, normal range) versus 37.7–37.9°C (upper normal). -

Investigating the Thermal Biology of Round Gobies by Justin Mark
Behavioural Thermoregulation and Escape Behaviour: Investigating the Thermal Biology of Round Gobies By Justin Mark Bridgeman Bachelor of Science, 2014, Brock University Submitted in partial fulfillment of the requirements for the degree of Master of Science Brock University, Faculty of Mathematics and Science Department of Biological Sciences St. Catharines, Ontario © 2019 Abstract The invasive round goby (Neogobius melanostomus) has successfully colonized all of the Great Lakes since its discovery in the region in 1991, yet little is known about its thermal biology. The focus of this thesis was to examine the effect of acclimation to unseasonably warm temperatures on round goby behavioural thermoregulation, as well as behavioural and physiological performance during escapes with warm acute temperatures. Juvenile gobies were acclimated to either 21°C or 24°C for each set of experiments. I first examined goby thermal preference in a shuttlebox through their ability to escape from unfavourable temperatures. I found that escape temperatures were plastic following acclimation to a rise in 3°C rise in temperature (+3°C) and associated positively with acclimation temperature, even though gobies showed slightly lower-than-expected average escape temperatures in each acclimation treatment. Interestingly, acclimation to +3°C leads to lower exploratory behaviour in warm waters and lower overall activity levels during behavioural thermoregulation. In risky situations involving threat of predation, exploratory behaviour is often linked to boldness. Next I investigated exploratory swimming through two behavioural traits: ability to voluntarily enter a tunnel and subsequent swimming activity while being chased in a detour task. Detour tasks require a fish to swim down a narrow space and then detour to the left or right as they approach a barrier. -

Mammalogy Lecture 17 – Thermoregulation/Water Balance I
Mammalogy Lecture 17 – Thermoregulation/Water Balance I. Introduction. Obviously, mammals are endotherms; they regulate body temperature via metabolic processes by burning energy. For all endotherms, there is a Thermal Neutral Zone When TA is low, energy is expended to keep warm. When TA is high, energy is expended to keep cool But for every endothermic species, there is a thermal neutral zone, the range of ambient temperatures across which there’s no higher cost of homeothermy. TLC - highest temperature at which an endotherm expends energy to stay warm TUC - lowest temperature at which an endotherm expends energy to stay cool Obviously, when ambient temperatures are either below TLC or above TUC, there is a metabolic cost to homeothermy (maintaining a constant body temperature). II. Adaptations for Cold – Temperatures in Zone A A. Large Size - B. High Basal Metabolic Rate - Cold adapted species have a higher than expected basal metabolic rate. For example, Red foxes, Vulpes vulpes, have a BMR that’s nearly twice as high as similar sized canids in warmer regions. C. Insulation - Pelage - forms a barrier of warm air next to the surface of the animal. Blubber - subcutaneous fat is commonly used as an insulating mechanism in marine mammals. D. Regional Heterothermy Extremities may be allowed to cool, sometimes to very low temperatures. This is accomplished by vasoconstriction. Urocitellus paryii - toe pads may be 2 o - 5 o C Ondatra zibethicus – extremities are allowed to cool to water temperature E. Systemic Heterothermy – Adaptive Hypothermia Characterized by: - Decreased heart rate - Vasoconstriction - severe reduction of blood flow to the extremities - Decreased breathing rate - Suppression of shivering - Decreased oxygen consumption (decreased metabolic rate) - Decreased body temperature There is usually great energy savings associated with hypothermia. -

The Evolution of Endothermy and Its Diversity in Mammals and Birds Author(S): Gordon C
Division of Comparative Physiology and Biochemistry, Society for Integrative and Comparative Biology The Evolution of Endothermy and Its Diversity in Mammals and Birds Author(s): Gordon C. Grigg, Lyn A. Beard, and Michael L. Augee Source: Physiological and Biochemical Zoology, Vol. 77, No. 6, Sixth International Congress of Comparative Physiology and Biochemistry Symposium Papers: Evolution and Advantages of Endothermy (November/December 2004), pp. 982-997 Published by: The University of Chicago Press. Sponsored by the Division of Comparative Physiology and Biochemistry, Society for Integrative and Comparative Biology Stable URL: http://www.jstor.org/stable/10.1086/425188 . Accessed: 08/11/2015 23:11 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. The University of Chicago Press and Division of Comparative Physiology and Biochemistry, Society for Integrative and Comparative Biology are collaborating with JSTOR to digitize, preserve and extend access to Physiological and Biochemical Zoology. http://www.jstor.org This content downloaded from 23.235.32.0 on Sun, 8 Nov 2015 23:11:10 PM All use subject to JSTOR Terms and Conditions 982 The Evolution of Endothermy and Its Diversity in Mammals and Birds Gordon C. Grigg1 thermy, including the capacity for homeothermic endothermy Lyn A. -

Temperature, Physiology, and the Ecology of Reptiles
Temperature, Physiology, and the Ecology of Reptiles RAYMOND B. HUEY Department of Zoology, University of Washington, Seattle, U.S.A. There is a great mass of information available concerning temperature as an ecological factor, but its actual operation is sometimes difficult to evaluate. A. HISTORICAL VIEWS OF TEMPERATURE AND REPTILIAN ECOLOGY The physiological differences of reptiles from other tetrapods have important ecological consequences. For example, their low metabolic rates (Bennett and Dawson, 1976) let reptiles drain environmental resources much less intensively than do birds and mammals. Their relatively impermeable integuments (Lillywhite and Maderson; Mautz, this volume) keep reptiles less closely constrained by hydric conditions than most amphibians (Kiester, 1971; Tracy, 1978; Nagy, this volume). These fundamental biological differences suggest that ecological and physiological studies of reptiles should complement rather than merely supplement parallel studies of other vertebrates (Pianka, 1977; Schoener, 1977; Regal, 1978; Schall and Pianka, 1978; A very, 1979; Pough, 1980, in press). The important discovery that desert reptiles behaviorally regulate body temperature was the first dramatic contribution of studies of reptiles to general ecological thought (Cowles and Bogert, 1944; Avery, this volume). By demonstrating that thermal homeostasis can be achieved by behavioral as well as by physiological adjustments and is a property of "lower" as well as of "higher" animals, this discovery revolutionized the philosophy and methodology of physiology and ecology. The demonstration that tempera- ture is intimately involved with the daily lives of reptiles, provided a unique focus for early ecological studies of reptiles. Indeed, for many years, few herpetologists departed for the field without several Schultheis ther- mometers! The concept that temperature is a fundamental factor in the ecology of Physiology. -

The Serotonin Syndrome
The new england journal of medicine review article current concepts The Serotonin Syndrome Edward W. Boyer, M.D., Ph.D., and Michael Shannon, M.D., M.P.H. From the Division of Medical Toxicology, he serotonin syndrome is a potentially life-threatening ad- Department of Emergency Medicine, verse drug reaction that results from therapeutic drug use, intentional self-poi- University of Massachusetts, Worcester t (E.W.B.); and the Program in Medical Tox- soning, or inadvertent interactions between drugs. Three features of the sero- icology, Division of Emergency Medicine, tonin syndrome are critical to an understanding of the disorder. First, the serotonin Children’s Hospital, Boston (E.W.B., M.S.). syndrome is not an idiopathic drug reaction; it is a predictable consequence of excess Address reprint requests to Dr. Boyer at IC Smith Bldg., Children’s Hospital, 300 serotonergic agonism of central nervous system (CNS) receptors and peripheral sero- 1,2 Longwood Ave., Boston, MA 02115, or at tonergic receptors. Second, excess serotonin produces a spectrum of clinical find- [email protected]. edu. ings.3 Third, clinical manifestations of the serotonin syndrome range from barely per- This article (10.1056/NEJMra041867) was ceptible to lethal. The death of an 18-year-old patient named Libby Zion in New York updated on October 21, 2009 at NEJM.org. City more than 20 years ago, which resulted from coadminstration of meperidine and phenelzine, remains the most widely recognized and dramatic example of this prevent- N Engl J Med 2005;352:1112-20. 4 Copyright © 2005 Massachusetts Medical Society. able condition. -
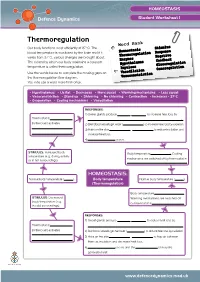
Thermoregulation Word Bank Our Body Functions Most Efficiently at 37°C
HOMEOSTASIS Student Worksheet 1 Thermoregulation Word Bank Our body functions most efficiently at 37°C. The Stimulus blood temperature is monitored by the brain and if it Homeostasis Response Thermoregulation varies from 37°C, various changes are brought about. Negative Enzymes The means by which our body maintains a constant feedback Hypothalamus Glucoregulation temperature is called thermoregulation. Impulses Osmoregulation Use the words below to complete the missing gaps on Vasodilation the thermoregulation flow diagram. Vasoconstriction You may use a word more than once. • Hypothalamus • Lie flat • Decreases • More sweat • Warming mechanisms • Less sweat • Vasoconstriction • Stand up • Shivering • No shivering • Contraction • Increases• 37ºC • Evaporation • Cooling mechanisms • Vasodilation RESPONSES: 1) Sweat glands produce more sweat to increase heat loss by Thermostat in . (in the brain) activates 2) Skin blood vessels get wider (vasodilatio n) to increase heat loss by radiation. 3) Hairs on the skin lie flat to reduce insulation and . increase heat loss. 4) No shivering occurs. STIMULUS: Increased body Body temperature decreases . Cooling temperature (e.g. during activity mechanisms are switched off by thermostat in or in hot surroundings). HOMEOSTASIS: Normal body temperature (37 ). Body temperature Normal body temperature (37 ). (Thermoregulation) Body temperature increas es. STIMULUS: Decreased Warming mechanisms are switched off body temperature (e.g. by thermostat in . in cold surroundings). RESPONSES: 1) Sweat glands produce more sweat to reduce heat loss by Thermostat in . (in the brain) activates 2) Skin blood vessels get narrower (vasodilatio n) to reduce heat loss by radiation. 3) Hairs on the skin lie flat to trap air between . them as insulation and decrease heat loss. -

Homeostasis: Thermoregulation
Homeostasis Draw a Block Diagram of a Homeostatic System. Homeostasis Input Set Point Change Output Difference Control Comparator Signal Effector Center Internal Measure of Output Draw a Block Diagram of a Homeostatic System. Homeostasis Input Set Point Comparator Change Output Difference Control Signal Effector Input Center Internal Measure of Output Output Sensor Homeostasis Input Set Point Change Control Center Signal Difference Increase effort + _ 0 Output Comparator Maintain effort + Effector _ Control Center Decrease effort Internal Measure of Output Homeostasis A C Input B Set Point D Change Output Difference Control Comparator Signal Effector Center Internal Measure of Output Which grouping represents the Nervous System? Homeostasis Sensory Systems Input Set Point Motor Systems Central Nervous System Change Output Difference Control Comparator Signal Effector Center Internal Measure of Output Homeostasis of thermoregulation Goals: To keep internal conditions constant Prevent the body from harmful effects of overheating or hypothermia Fever is part of the innate, non-specific immune response, that helps to create a hostile enviroment for microbial pathogens Homeostasis of thermoregulation Heat transfer from body Radiation – emission of the electromagnetic waves, all matter possesses that have temperature above absolute zero Convection – energy transfer between objects that are in physical contact (a body and a chair) Conduction – energy transfer between body and its enviroment, due to fluid motion Evaporation – most effective. In hot weather (x humidity of the enviroment) Heat production Most produced in deep organs: liver, brain, heart (heat is the byproduct of metabolic reactions) and in contraction of muscles Homeostasis of thermoregulation Input Autonomic Set Point Endocrine Th. receptors HTH HTH (posterior part) HTH (preoptic and its projections Motor area) to various brain regions Behavioral Change Output Comparator Difference Control Signal Effector HTH Center Body temp. -

Somatic Influences on Subjective Well
REVIEW ARTICLE published: 13 January 2015 doi: 10.3389/fpsyg.2014.01580 Somatic influences on subjective well-being and affective disorders: the convergence of thermosensory and central serotonergic systems Charles L. Raison 1, Matthew W. Hale 2, Lawrence E. Williams 3,TorD.Wager4 and Christopher A. Lowry 5* 1 Department of Psychiatry, Norton School of Family and Consumer Sciences, College of Medicine, College of Agriculture and Life Sciences, University of Arizona, Tucson, AZ, USA 2 Department of Psychology, School of Psychological Science, La Trobe University, Bundoora, Australia 3 Marketing Division, Leeds School of Business, University of Colorado Boulder, Boulder, CO, USA 4 Department of Psychology and Neuroscience, University of Colorado Boulder, Boulder, CO, USA 5 Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO, USA Edited by: Current theories suggest that the brain is the sole source of mental illness. However, Mattie Tops, VU University affective disorders, and major depressive disorder (MDD) in particular, may be better Amsterdam, Netherlands conceptualized as brain-body disorders that involve peripheral systems as well. This Reviewed by: perspective emphasizes the embodied, multifaceted physiology of well-being, and Mattie Tops, VU University Amsterdam, Netherlands suggests that afferent signals from the body may contribute to cognitive and emotional Fieke Maria Antoinet Wagemans, states. In this review, we focus on evidence from preclinical and clinical studies suggesting Tilburg University, Netherlands that afferent thermosensory signals contribute to well-being and depression. Although Sayamwong E. Hammack, thermoregulatory systems have traditionally been conceptualized as serving primarily University of Vermont, USA homeostatic functions, increasing evidence suggests neural pathways responsible for *Correspondence: Christopher A.