Preparation Range Cotton and Cotton Blend
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ballistic Materials Handbook
Ballistic materials handbook Aramids by Teijin 2 Aramids by Teijin Handbook ballistic materials 3 Teijin Aramid and ballistic protection The intensity of threatening environments for law enforcement, emergency responders and defense forces around the world is becoming higher and the people operating in these hostile environments need to take greater care than ever. This growing threat of violence has led to an increasing demand for ballistic protection. At Teijin Aramid we are dedicated to providing this protection with our high performance para-aramid fiber Twaron® and UHMWPE Endumax® film. With excellent energy absorption Index properties, tenacity and impact resistance, Twaron® and Endumax® offer effective and comfortable ballistic protection Teijin Aramid and ballistic protection 2 solutions with an outstanding cost-performance ratio. In the Twaron® ballistic yarns 4 more than 30 years that Twaron® has been available on the Twaron® ballistic fabrics 7 market, it has helped to save thousands of lives worldwide. Ballistic laminates & coated fabrics 12 Key applications for Twaron® and Endumax® include bullet/ Uni-directional laminates 17 fragment/stab/spike resistant vests, helmets and ballistic Ballistic prepregs 19 protection of vehicles, aircrafts and vessels. Cross sections 21 Endumax® Shield 22 2 Aramids by Teijin Handbook ballistic materials 3 Soft ballistic protection The threats to modern armies and law enforcement forces have multiplied, creating the need for protection from all kinds of bullets and fragments as well as stabbing with sharp objects. And these days it’s not only soldiers and policemen who are facing increased threats; prison guards, cash carriers and private individuals also need to be protected. -

FL.Datasheet Kevlar® Distribution Program.Indd
MOVING HIGH PERFORMANCE FIBERS FORWARD KEVLAR® DISTRIBUTION PROGRAM FIBERS PROCESSES PRODUCTS WHY FIBER-LINE® DUPONTFIBER TM OPTICAL DISTRIBUTION CABLES PROGRAM? Key Features FIBER-LINE® values its relationships with both its customers and • Purchase small quantities of Kevlar® suppliers. Over the past several years, FIBER-LINE® and DuPontTM have Para-Aramid formed a strong partnership based upon the synergies between both • Many deniers & types available organizations. • Customize your Kevlar® solution with FIBER-LINE® performance adding processes FIBER-LINE®’s ability to add value to the already attractive properties of both Kevlar® Para-Aramid & Nomex® Meta-Aramid creates more opportunity in the market place to provide solution driven products to a diverse range of markets. Because FIBER-LINE® already processes so many different types and deniers of both Kevlar® & Nomex®, FIBER-LINE® have been authorized by DuPontTM to distribute small quantities of these fibers to an ever- growing customer base. Through this program, we hope to introduce businesses of all sizes to the benefit of aramid fibers. Contact us today for small order quantity orders. Available Deniers 200, 380, 400, 750AP, 800AP, 1000, 1000AP, 1420, 1500, 1500AP, 1500BK(Black), 2160, 2250, 2840, 3000, 7100. MOVING HIGH PERFORMANCE FIBERS FORWARD KEVLAR® PARA-ARAMID (HM) BARE FIBER PERFORMANCE Chemical Chemical Chemical Abrasion Yarn on Yarn Ultraviolet (UV) Flame Resistance Resistance Resistance Resistance Abrasion Resistance Resistance (Acid) (Alkali) (Organic Solvent) P O X P P P P CHEMICAL COMPATIBILITY Chemical Resistance to Acid: Degrades in Formic, Hydrochloric, and Sodium Hydroxide acid. Chemical Resistance to Alkali: Strong alkalis will attack at high temperature or concentration. Chemical Resistance to Organic Solvent: Degrades moderately in Carbon Tetrachloride and Ethylene Glycol/Water. -

Endumax® – an Ultra-Strong Thin Film with a High Modulus Contents
Endumax® – an ultra-strong thin film with a high modulus Contents What is Endumax? 3 How is Endumax produced? 4 What types of Endumax are available? 6 What can Endumax be used for? 8 Endumax – a unique combination of properties 10 About Teijin Teijin is a technology-driven global group, based in Japan, offering advanced solutions in the areas of sustainable transportation, information and electronics, safety and protection, environment and energy, and healthcare. Its main fields of operation are high-performance fibers (e.g., aramid, carbon fibers and composites), healthcare, films, resin & plastic processing, polyester fibers, product conversion and IT. The group has some 150 companies and around 17,000 employees spread over 20 countries worldwide. Endumax is part of Teijin’s high- performance fibers business, which also produces the aramid fibers Twaron, Technora and Teijinconex. Teijin’s high-performance fibers business is based in Arnhem, The Netherlands. 2 What is Endumax? Endumax film is a new, patented high-performance film developed and made by Teijin. It can be used in a wide variety of products for various market segments – anywhere, in fact, where there is a need for superior strength, safety, light weight or durability. For example, Endumax is used worldwide in applications and markets ranging from ballistic protection (armoring and bulletproof vests), ropes and cables to cargo containers, laminated sails and even loudspeakers. The film shape of Endumax allows for easy processing and seamless integration into the application of the customer. Super-strong and more Weight-for-weight, Endumax is 11 times stronger than steel. But Endumax offers more than incredible strength. -

Annex 2B Tariff Schedule of the United States See General Notes to Annex 2B for Staging Explanation HTSUS No
Annex 2B Tariff Schedule of the United States See General Notes to Annex 2B for Staging Explanation HTSUS No. Description Base Rate Staging 0101 Live horses, asses, mules and hinnies: 0101.10.00 -Purebred breeding animals Free E 0101.90 -Other: 0101.90.10 --Horses Free E 0101.90.20 --Asses 6.8% B --Mules and hinnies: 0101.90.30 ---Imported for immediate slaughter Free E 0101.90.40 ---Other 4.5% A 0102 Live bovine animals: 0102.10.00 -Purebred breeding animals Free E 0102.90 -Other: 0102.90.20 --Cows imported specially for dairy purposes Free E 0102.90.40 --Other 1 cent/kg A 0103 Live swine: 0103.10.00 -Purebred breeding animals Free E -Other: 0103.91.00 --Weighing less than 50 kg each Free E 0103.92.00 --Weighing 50 kg or more each Free E 0104 Live sheep and goats: 0104.10.00 -Sheep Free E 0104.20.00 -Goats 68 cents/head A 0105 Live poultry of the following kinds: Chickens, ducks, geese, turkeys and guineas: -Weighing not more than 185 g: 0105.11.00 --Chickens 0.9 cents each A 0105.12.00 --Turkeys 0.9 cents each A 0105.19.00 --Other 0.9 cents each A -Other: 0105.92.00 --Chickens, weighing not more than 2,000 g 2 cents/kg A 0105.93.00 --Chickens, weighing more than 2,000 g 2 cents/kg A 0105.99.00 --Other 2 cents/kg A 0106 Other live animals: -Mammals: 0106.11.00 --Primates Free E 0106.12.00 --Whales, dolphins and porpoises (mammals of the order Cetacea); manatees and dugongs (mammals of the order Sirenia) Free E 0106.19 --Other: 2B-Schedule-1 HTSUS No. -

OCTOBER ICC MEETING 10 OCTOBER 2018 Blank Page
OCTOBER ICC MEETING 10 OCTOBER 2018 Blank Page Meeting agenda AWI Woolgrower Industry Consultative Committee (ICC) Wednesday, 10 October 2018, 8.00am – 3.00pm AWI’s Sydney Office, Level 6, 68 Harrington St, The Rocks Dinner: 7pm, 9th October at Endeavour Tap Rooms – 39/43 Argyle St, The Rocks NSW 2000 (The Rocks) The purpose of AWI’s ICC is to enable AWI to formally consult with woolgrower representative organisations, allowing them to provide feedback on priorities from their members, and for AWI to report on its performance and plans. These priorities guide AWI’s investment and activities. SESSION 1: Your members’ priorities for AWI TIME AGENDA ITEM DISCUSSION LEAD 8:00 Breakfast 8.30 – 8.45 1 Welcome and general business Wal Merriman 1.1 Review minutes from previous meeting 1.2 Review actions from last meeting 8.45 – 10.05 2 ICC members report on their members’ R&D and ICC members marketing priorities for AWI (10 mins 2.1 Australian Association of Stud Merino Breeders each member 2.2 Australian Superfine Woolgrowers Association and 20 mins 2.3 Australian Wool Growers Association for guest 2.4 Broad wool breeds participant) 2.5 Pastoralists and Graziers Association of WA 2.6 WoolProducers Australia 2.7 Liebe Group 10.05 – 10.35 3 ICC member agenda items – request for briefing ICC Members from AWI on issues 3.1 Australian Superfine Wool Growers’ Association 3.2 Australian Wool Growers’ Association 3.3 WoolProducers Australia 10.35 – 10.50 Morning tea 10.50 – 11.20 4 Update from Department of Agriculture, Water Michael Ryan and Resources -

High Performance Fibers
High Performance Fibers One step ahead High Performance Fibers are engineered for extreme uses; whether the requirement is exceptional strength, stiffness, heat resistance and/or chemical resistance. EuroFibers is proud distribution partner of the leading brands in this industry with the ability to tailor these tough fibers to the need of our customers, whether it be coating, twisting, assembling, plying or cutting. HMPE Fiber Para-Aramid Fiber Ultra high molecular weight polyethylene (UHMwPE), high modulus Para-aramid fibers are a class of heat-resistant and extremely strong polyethylene (HMPE) or high performance polyethylene fibers (HPPE) synthetic fibers. The ultimate strength of some aramid fibers can are extremely strong and are the lightest of all ultra-strong fibers. exceed 3500 MPa. Aramid has an outstanding strength-to-weight The ultimate strength can exceed 3000 MPa. However, due to its low ratio, even better than carbon, and excellent dimensional stability melting point of about 150°C (295°F) they are not suitable for elevated due to the high young’s modulus. Para-aramid has a decomposition temperature applications. The fiber is mainly used in protective temperature of ± 500 ºC. Technora® is a para-aramid fiber made clothing like ballistic vests, helmets, cut-resistant glove and tension from copolymers and is produced in the different process from PPTA members like ropes, slings and fishing lines. (poly-paraphenylene terephthalamide). EuroFibers is the premium distributor of DSM offering the extensive EuroFibers is the premium distributor of Teijin® offering their Dyneema® and Trevo® portfolio to our customer base. exceptional aramid fibers Twaron® and Technora® to a wide variety of customers. -

401 Part 73—Listing of Color Ad- Ditives Exempt from Certifi- Cation
Food and Drug Administration, HHS Pt. 73 the act, has notified the sponsor or in- 73.352 Paracoccus pigment. vestigator that the proposed disposi- 73.355 Phaffia yeast. tion for food is authorized. Any person 73.450 Riboflavin. 73.500 Saffron. who contests a refusal to grant such 73.530 Spirulina extract. authorization shall have an oppor- 73.575 Titanium dioxide. tunity for a regulatory hearing before 73.585 Tomato lycopene extract; tomato ly- the Food and Drug Administration pur- copene concentrate. suant to part 16 of this chapter. 73.600 Turmeric. (b) The person who introduced such 73.615 Turmeric oleoresin. shipment or who delivers the color ad- ditive or a food, drug, or cosmetic con- Subpart B—Drugs taining such an additive into interstate 73.1001 Diluents in color additive mixtures commerce shall maintain adequate for drug use exempt from certification. records showing the name and post-of- 73.1010 Alumina (dried aluminum hydrox- fice address of the expert to whom the ide). color additive is shipped, date, quan- 73.1015 Chromium-cobalt-aluminum oxide. tity, and batch or code mark of each 73.1025 Ferric ammonium citrate. 73.1030 Annatto extract. shipment and delivery for a period of 2 73.1070 Calcium carbonate. years after such shipment and delivery. 73.1075 Canthaxanthin. Upon the request of a properly author- 73.1085 Caramel. ized employee of the Department, at 73.1095 b-Carotene. reasonable times, he shall make such 73.1100 Cochineal extract; carmine. records available for inspection and 73.1125 Potassium sodium copper copying. chlorophyllin (chlorophyllin-copper com- plex). -
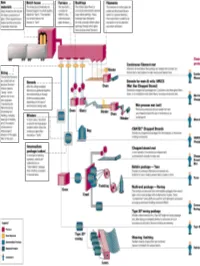
All About Fibers
RawRaw MaterialsMaterials ¾ More than half the mix is silica sand, the basic building block of any glass. ¾ Other ingredients are borates and trace amounts of specialty chemicals. Return © 2003, P. Joyce BatchBatch HouseHouse && FurnaceFurnace ¾ The materials are blended together in a bulk quantity, called the "batch." ¾ The blended mix is then fed into the furnace or "tank." ¾ The temperature is so high that the sand and other ingredients dissolve into molten glass. Return © 2003, P. Joyce BushingsBushings ¾The molten glass flows to numerous high heat-resistant platinum trays which have thousands of small, precisely drilled tubular openings, called "bushings." Return © 2003, P. Joyce FilamentsFilaments ¾This thin stream of molten glass is pulled and attenuated (drawn down) to a precise diameter, then quenched or cooled by air and water to fix this diameter and create a filament. Return © 2003, P. Joyce SizingSizing ¾The hair-like filaments are coated with an aqueous chemical mixture called a "sizing," which serves two main purposes: 1) protecting the filaments from each other during processing and handling, and 2) ensuring good adhesion of the glass fiber to the resin. Return © 2003, P. Joyce WindersWinders ¾ In most cases, the strand is wound onto high-speed winders which collect the continuous fiber glass into balls or "doffs.“ Single end roving ¾ Most of these packages are shipped directly to customers for such processes as pultrusion and filament winding. ¾ Doffs are heated in an oven to dry the chemical sizing. Return © 2003, P. Joyce IntermediateIntermediate PackagePackage ¾ In one type of winding operation, strands are collected into an "intermediate" package that is further processed in one of several ways. -

The World of Teijin Aramid
Teijin Aramid @ Techtextile Middle East Symposium Dubai , 20th Feburary 2014 René Lohmann Sales & Marketing Ballistics Teijin Aramid GmbH, Wuppertal, Germany Agenda • Global Key Trends • Aramids in the middle East • Stopping the bullet • 550dtex f1000 ballistic yarn • LFT SB1 Plus • SRM • Microflex • Twaron and Endumax in helmets • Our research capabilities • Sustainable strength Global Key Trends Global key trends • In recent years, there have been significant changes in the requirements placed on both consumer and industrial goods around the world • There is a growing demand for products that combine high performance with durability and low maintenance • At the same time, these products need to be cost-effective, use less energy, enhance safety, and they should ideally have a smaller lifecycle ecological footprint Sharing our customers’ ambitions • Our prime aim is to add value to the bottom line of our customers • Co-creation and open innovation with customers on advanced products and applications • Loyalty to customers • Long-term relationships • Sharing knowledge & expertise Global presence Aramid in the middle East Our product portfolio Para-aramid • Twaron • Technora Meta-aramid • Teijinconex Poly-ethylene • Endumax Different types to fit application requirements Twaron Technora Short-cut fiber Staple fiber Pulp Fabrics Tape Powder Short-cut fibers Endumax , UHMWPE Tape and X-ply • Ropes, cables and slings • Ballistic protection • Robotics / Force transmission Technora, for enhanced properties • High tensile strength • Weight for -

Reliable Long-Haul OFC Networks
Reliable long-haul OFC networks With more and more data being transmitted over longer The capability of Twaron UP is further enhanced by the high distances and at ever-greater transmission speeds, tenacity of the product. As a result, your cables will be lighter reliable high-speed connectivity is essential. For long- at the same span. If circumstances require, you can also span haul OFC networks, ADSS cable manufacturers therefore longer distances than before. focus on maximum span length, minimum sag, and maximum wind and ice load resistance. In addition, their optical fiber cables need to be able to deal with variable Key benefits of usingTwaron UP environmental conditions and should be easy to install, even when live-line installations are required. • Significant reduction of total cable costs At Teijin Aramid, we have more than two decades of • Lower cable weight at the same span experience in developing innovative aramid fiber products that strengthen and protect optical fiber cables. Our latest • Longer spans possible high-performance lightweight aramid fiber Twaron Ultimate Performance (Twaron UP) offers the highest modulus in • Sustainable solution the para-aramid industry, some 20% higher than what is currently offered by our competitors. This means less aramid fiber material is needed to achieve the same performance. Exceptional dimensional stability Twaron UP’s extra-high modulus means that the dimensional stability of the yarn is some 20% better than that of yarns offered by the competition. The higher the modulus of the aramid fiber, the lower the creep in the course of time. This means you can count on the material being highly durable. -

Mechanical Behaviour of Aramid and Glass Fibre Reinforced Polyester Resin Composite
Volume 5, Issue 4, April – 2020 International Journal of Innovative Science and Research Technology ISSN No:-2456-2165 Mechanical Behaviour of Aramid and Glass Fibre Reinforced Polyester Resin Composite G. Dhanasekar 1, P. Prema2 1 PG Scholar , 2Assistant Professor Dept. of Mechanical Engg., Alagappa Chettiar Government College of Engineering and Technology, Karaikudi - 630003, Tamil Nadu, INDIA Abstract:- In today’s fast-moving world, vehicles form bumper system which differ across nations. In European an indispensable part of human life. The yearly vehicle nations, pendulum test is the standards for the low-impact creation pace of the world is assessed to arrive at 100 test which is set at 2.5mph (4.0 km/hr) and guarantees no million constantly in the year 2030. New guidelines are harm to the bumper beam though in nations like the USA being made to think about the potential effect of the and North America, a similar test is done at 5mph (8 km/hr) utilization of vehicles in the environment. The End of in which the harm to the fascia is not considered. The real Life Vehicles (ELV) regulations attempt to encourage impact damping behavior in an automotive bumper system car manufacturers to shift to the use of polymer based includes various parameters such as quantity, direction, materials. The hybridization of engineered fiber contact area and position of the applied load and so it is (Aramid fiber and glass fiber) gives a technique to complex to analyze it M.M. Davoodi [1]. This project work improve the mechanical properties over common replaces the combinations with polymeric composite filaments. -

(12) United States Patent (10) Patent No.: US 8,133,584 B2 Zhu (45) Date of Patent: Mar
USOO8133584B2 (12) United States Patent (10) Patent No.: US 8,133,584 B2 Zhu (45) Date of Patent: Mar. 13, 2012 (54) CRYSTALLIZED META-ARAMID BLENDS 3,673,143 A 6, 1972 Bair et al. FOR FLASH FIRE AND ARC PROTECTION 3.3 A 2. E. lis HAVING IMPROVED COMFORT 3,819,587 A 6, 1974 Kwoleck 3,869,429 A 3, 1975 Blades (75) Inventor: Reiyao Zhu, Moseley, VA (US) 4,172,938 A 10/1979 Mera et al. 4,612,150 A 9, 1986 DeHowitt (73) Assignee: E.I. du Pont de Nemours and 4,668,234 A 5, 1987 Vance et al. Company, Wilmington, DE (US) 4,755,335 A T. 1988 Ghorashi 4,883,496 A 11, 1989 Ghorashi (*) Notice: Subject to any disclaimer, the term of this 39: A 2: ER, al. patent is extended or adjusted under 35 5,506,042 A 4/1996 Ichibori et al. U.S.C. 154(b) by 16 days. 7,156,883 B2 * 1/2007 Lovasic et al. ............... 8, 11551 7,348,059 B2 3/2008 Zhu et al. 2005/0O25963 A1 2, 2005 Zhu (21) Appl. No.: 12/756,513 2010/01 12312 A1* 5, 2010 Tutterow et al. .............. 428,196 (22) Filed: Apr. 8, 2010 * cited by examiner (65) Prior Publication Data Primary Examiner — Jennifer Chriss US 2011 FO250810 A1 Oct. 13, 2011 Assistant Examiner — Ricardo E. Lopez (51) Int. Cl. (57) ABSTRACT B32B 27/4 (2006.01) A yarn, fabric, and garment Suitable for use in arc and flame D02G 3/00 (2006.01) protection and having improved flash fire protection consist DO3D 5/2 (2006.01) ing essentially of from (a) 50 to 80 weight percent meta D04H I3/00 (2006.01) aramid fiber having a degree of crystallinity of at least 20%, (52) U.S.