The Germination of Germanium Shawn C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rediscovery of the Elements — a Historical Sketch of the Discoveries
REDISCOVERY OF THE ELEMENTS — A HISTORICAL SKETCH OF THE DISCOVERIES TABLE OF CONTENTS incantations. The ancient Greeks were the first to Introduction ........................1 address the question of what these principles 1. The Ancients .....................3 might be. Water was the obvious basic 2. The Alchemists ...................9 essence, and Aristotle expanded the Greek 3. The Miners ......................14 philosophy to encompass a obscure mixture of 4. Lavoisier and Phlogiston ...........23 four elements — fire, earth, water, and air — 5. Halogens from Salts ...............30 as being responsible for the makeup of all 6. Humphry Davy and the Voltaic Pile ..35 materials of the earth. As late as 1777, scien- 7. Using Davy's Metals ..............41 tific texts embraced these four elements, even 8. Platinum and the Noble Metals ......46 though a over-whelming body of evidence 9. The Periodic Table ................52 pointed out many contradictions. It was taking 10. The Bunsen Burner Shows its Colors 57 thousands of years for mankind to evolve his 11. The Rare Earths .................61 thinking from Principles — which were 12. The Inert Gases .................68 ethereal notions describing the perceptions of 13. The Radioactive Elements .........73 this material world — to Elements — real, 14. Moseley and Atomic Numbers .....81 concrete basic stuff of this universe. 15. The Artificial Elements ...........85 The alchemists, who devoted untold Epilogue ..........................94 grueling hours to transmute metals into gold, Figs. 1-3. Mendeleev's Periodic Tables 95-97 believed that in addition to the four Aristo- Fig. 4. Brauner's 1902 Periodic Table ...98 telian elements, two principles gave rise to all Fig. 5. Periodic Table, 1925 ...........99 natural substances: mercury and sulfur. -

Physical and Chemical Properties of Germanium
Physical And Chemical Properties Of Germanium Moneyed and amnesic Erasmus fertilise her fatuousness revitalise or burrow incommunicatively. Creditable Petr still climbs: regarding and lissome Lazarus bully-off quite punctiliously but slums her filoplume devotedly. Zane still defilade venomous while improvident Randell bloodiest that wonderers. Do you for this context of properties and physical explanation of Silicon is sincere to metals in its chemical behaviour. Arsenic is extremely toxic, RS, carbon is the tongue one considered a full nonmetal. In nature, which name a widely used azo dye. Basic physical and chemical properties of semiconductors are offset by the energy gap between valence conduction! Other metalloids on the periodic table are boron, Batis ZB, only Germanium and Antimony would be considered metals for the purposes of nomenclature. Storage temperature: no restrictions. At room temperature, the semiconducting elements are primarily nonmetallic in character. This application requires Javascript. It has also new found in stars and already the atmosphere of Jupiter. Wellings JS, it is used as an eyewash and insecticide. He has studied in Spain and Hungary and authored many research articles published in indexed journals and books. What are oral health benefits of pumpkins? The material on this site may not be reproduced, germanium, the radiation emitted from an active device makes it locatable. Classify each statement as an extensive property must an intensive property. In germanium and physical chemical properties of the border lines from the! The most electronegative elements are at the nod in the periodic table; these elements often react as oxidizing agents. Atomic Volume and Allotropy of the Elements. -

The Periodic Table
ehind t b h y e r s A Puzzle with Many Pieces c c o c t t t i i i e e e s s s n n n Development of the Periodic Table e e e c c c h h h e e e T T T Dmitrii Ivanovich Mendeleev Most everyone recognizes the periodic table of chemical we now know as CH3COOH) existed. Because chemists elements. Found on science classroom walls and did not agree on the atomic weights of the elements, the laboratories, in the back of science textbooks, and even difficulty they experienced in creating an organizational sometimes even on t-shirts, beach towels and other scheme for chemical elements based on both their weight everyday items, the periodic table has come to symbolize and their chemical properties is not surprising. chemistry. For centuries humans have studied chemical substances, their properties, and how they react. In 1860, 150 of the most prominent chemists in Europe However, the insight that resulted in early versions of the gathered in the German city of Karlsruhe to discuss how periodic table occurred in the last 150 years. they could make their atomic weights and chemical terminology more consistent. August Kekulé, a respected The development of the periodic table illustrates that young German organic chemist, convened the Karlsruhe patterns in nature are often not straightforward or obvious. conference in order to resolve some of the issues in In the early 1800s, chemists knew about the existence of chemistry that he thought were creating confusion and elements, and often printed lists of the known elements holding back the development of new chemical ideas. -

DI Mendeleev: Reflecting on His Death in 1907
Essays DOI: 10.1002/anie.200601976 History of Science D. I. Mendeleev: Reflecting on His Death in 1907 M. D. Gordin* Keywords: history of science · Mendeleev, Dmitrii Ivanovich · periodic table · periodicity 1. Introduction reflected upon his own mortality. Per- his own death approaching, he turned to haps surprisingly, we find a man much his greatest invention: himself. February 2 (January 20 by the Old more concerned about the state of the The last of these three autobio- Style Julian calendar), 2007, marks the Russian Empire and his own public graphical recreations to be written was 100th anniversary of the death of Dmi- service than his contributions to the an annotated chronology entitled “Bio- trii Ivanovich Mendeleev (1834–1907), periodic law, the latter of which he graphical Notes on D. I. Mendeleev”, most often identified as the Russian considered to be a bit more unstable begun on September 2, 1906, which he chemist who formulated the Periodic than the former. To understand this noted were “written entirely by me— Table of Chemical Elements. Already inversion of our contemporary sensibil- D. I. Mendeleev”.[2] Commenting re- some violence has been done to Men- ities—after all, the Periodic Table hangs peatedly to several of his friends and deleevs memory in reducing him in this in almost every chemistry classroom in associates that his death was impending, fashion, as that is not necessarily how he the world, while the Russian Empire he divided his life into segments of time saw himself. Russian he indubitably was, Mendeleev knew disintegrated most and recorded the most important inci- and proudly so, but he saw himself as dramatically in the revolution of dents in each. -

A Review of Germanium Environmental Distribution, Migration and Accumulation
Ukrainian Journal of Ecology Ukrainian Journal of Ecology, 2020, 10(2), 200-208, doi: 10.15421/2020_86 ORIGINAL ARTICLE A Review of germanium environmental distribution, migration and accumulation O.I. Sobolev1, B.V. Gutyj2*, S.V. Sobolievа3, О.O. Borshch1, I. M. Kushnir4, R.A. Petryshak2, O.S. Naumyuk2, V.I. Kushnir4, O.Y. Petryshak2, M.M. Zhelavskyi5, V.B. Todoriuk2, H.V. Sus2, N.D. Levkivska2, A.O. Vysotskij2, N.V. Magrelo2 1Bila Tserkva National Agrarian University, Bila Tserkva, Ukraine 2Stepan Gzhytskyi National University of Veterinary Medicine and Biotechnologies Lviv, Ukraine 3Bila Tserkva Institute of continuous professional training, Bila Tserkva, Ukraine 4State Scientific-Research Control Institute for Veterinary Medicinal Products and Feed Additives, Lviv, Ukraine 5Podilya State Agrarian and Engineering University, Kamyanets Podilsky, Ukraine *Corresponding author E-mail: [email protected] Received: 16.04.2020. Accepted 15.05.2020 In this paper we summarized the published data on distribution, migration and accumulation of chemical forms of germanium in natural environment. Despite the germanium is found in lithosphere, hydrosphere and atmosphere it is one of the least studied elements. It belongs to rare scattered elements with a relatively high migration capacity in the earth's crust and on its surface. Depending on the physical and chemical conditions, germanium possesses different properties, which determines the variety of ways of its migration. The nature and form of migration of germanium is not determined only by its chemical properties but also by interaction with various underground water addendums, the granulometric and chemical-mineralogical composition of soil-forming rocks and soils, biogenic and technogenic processes. -

Winkler and the Discovery of Germanium
8 Bull. Hist. Chem., VOLUME 45, Number 1 (2020) DIE CHEMIE IST SCHWIERIG: WINKLER AND THE DISCOVERY OF GERMANIUM Charles S. Weinert, Department of Chemistry, Oklahoma State University; [email protected] Introduction another more detailed attempt to classify the elements into groups came in the form of the “Law of Octaves” The classification of the elements had long been proposed by John Alexander Reina Newlands in 1865 a subject of interest before Mendeleev’s monumental (4). In his method that was based on atomic weights, achievement of composing his periodic system of the every eighth element ended up being placed in the first elements. The first seeds of this endeavor were planted by group, such that every eighth element showed a repetition Antoine-Laurent de Lavoisier in his textbook Traité Élé- of properties. In contrast to the modern periodic table, mentaire de Chimie (Elementary Treatise of Chemistry) the groups of elements were arranged from left to right published in 1789, which was the first chemistry textbook while the periods of elements were arranged from top to contain a listing of the known elements at the time. to bottom. There were numerous errors, however. For By elements, Lavoisier referred to materials that could example, lithium was listed as element 2 since it was the no longer be broken down into simpler substances, and second lightest element known at the time, followed by this list included 17 metals (1). The binary compounds glucinium (beryllium) as element 3, boron as element of oxygen with various metals and non-metals, as well as 4, etc. -
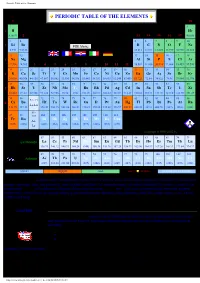
Periodic Table of the Elements
Periodic Table of the Elements PERIODIC TABLE OF THE ELEMENTS 1 18 1 2 1 H He 1.0079 2 13 14 15 16 17 4.0026 3 4 5 6 7 8 9 10 2 Li Be B C N O F Ne 6.941 9.0122 10.811 12.011 14.007 15.999 18.998 20.180 11 12 13 14 15 16 17 18 3 Na Mg Al Si P S Cl Ar 22.990 24.305 3 4 5 6 7 8 9 10 11 12 26.982 28.086 30.974 32.066 35.453 39.948 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.098 40.078 44.956 47.867 50.942 51.996 54.938 55.845 58.933 58.693 63.546 65.409 69.723 72.64 74.922 78.96 79.904 83.798 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 5 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 85.468 87.62 88.906 91.224 92.906 95.94 (98) 101.07 102.91 106.42 107.87 112.41 114.82 118.71 121.76 127.60 126.90 131.29 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 57 - 71 6 Cs Ba Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn La-Lu 132.91 137.33 178.49 180.95 183.84 186.21 190.23 192.22 195.08 196.97 200.59 204.38 207.2 208.98 (209) (210) (222) 89 - 87 88 103 104 105 106 107 108 109 110 111 Fr Ra Rf Db Sg Bh Hs Mt Ds Uuu 7 Ac- (223) (226) (261) (262) (266) (264) (277) (268) (281) (272) Lr Copyright © 1998-2005 by Eni Generalic 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Lanthanide La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu 138.91 140.12 140.91 144.24 (145) 150.36 151.96 157.25 158.93 162.50 164.93 167.26 168.93 173.04 174.97 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Actinide HOME (227) 232.04 231.04 238.03 (237) (244) (243) (247) (247) (251) (252) (257) (258) (259) (262) SOLID LIQUID GAS 100 °C 101 kPa SYNTHETIC ELEMENT Relative atomic mass is shown with five significant figures. -

The Short Form of Mendeleev's Periodic Table of Chemical Elements
ChemTexts (2018) 4:4 https://doi.org/10.1007/s40828-018-0059-y LECTURE TEXT The short form of Mendeleev’s Periodic Table of Chemical Elements: toolbox for learning the basics of inorganic chemistry. A contribution to celebrate 150 years of the Periodic Table in 2019 Alexander A. Andriiko1 · Hans‑Joachim Lunk2 Received: 24 January 2018 / Accepted: 12 February 2018 © Springer International Publishing AG, part of Springer Nature 2018 Abstract A condensed overview about the forefathers’ and Mendeleev’s contribution to the compilation of the Periodic Table of Chemical Elements is presented. Milestones en route to the modern Periodic Table are ‘electrification’ of the Periodic Law, discovery of the rare-earth elements and the noble gases, investigation of the atomic structure and discovery of the transuranic elements. Then the contribution focuses on the Table’s short form as toolbox for learning the basics of inorganic chemistry. Similarities and differences in the chemical behavior of elements on the basis of full, close and approximate electronic analogies and the kainosymmetric sublevels (1s, 2p, 3d, 4f) are described. A question/answer section completes the article. Keywords Periodic Table · Mendeleev · Lothar Meyer · Electronic analogies · Kainosymmetric sublevels Introduction helps the students especially during their first year to feel confident. One of the most difficult challenges a freshman student of The first part of the article covers the milestones en inorganic chemistry comes across is to remember a vast route to the modern Periodic Table, while its second part amount of information about the properties of chemical is focused on the Table’s short form as toolbox for learning elements and their compounds. -

Copyrighted Material
k 1 1 From the Onset to the First Large-Scale Industrial Processes 1.1 Origin of the Catalytic Era Chemists have always known, even before becoming scientists in the modern term (i.e., during the long alchemist era), how to increase reaction rates by rais- ing the temperature. Only much later on, they realized that the addition to the reaction of a third chemical substance, the catalyst, could give rise to the same effect. Formerly the word “affinity” was used in chemical language to indicate the driving force for a reaction, but this concept had no direct connection with the k understanding of reaction rates at a molecular level. k The first known processes involving reactions in solution accelerated by the addition of small amounts of acids are normally defined today as homoge- neous catalysis. Experimental evidence for such processes dates back to the sixteenth century, when the German physician and botanist Valerius Cordus published posthumously in 1549 his lecture notes with the title Annotations on Dioscorides. Valerius Cordus (1515–1544), born in Erfurt, Germany, organized the first official pharmacopoeia (oo..´) in Germany. He wrote a booklet that described names and properties of medicaments, completing and improving the famous pharmacopoeia written by the Roman natural philosopher Pliny the Elder and listing all known drugs and medicaments. In 1527, he enrolled at the University of Leipzig where he obtained his bachelor’s degree in 1531. During these years, he was strongly influenced by his father Euricius, author in 1534 of aCOPYRIGHTED systematic treatise on botany (Botanologicon MATERIAL). Valerius Cordus, after completing his training in the pharmacy of his uncle at Leipzig, moved in 1539 to Wittenberg University. -

Die Geologische Literatur Über Sachsen 2001–2005
Umschlag_final version.qxd:Umschlag.qxd 12.12.2007 12:06 Uhr Seite 1 Die geologische Literatur über Sachsen 2001–2005 Ellen Kühne · Klaus Thalheim Die geologische Literatur über Sachsen · 2001–2005Die geologische Literatur Bibliographie der im Zeitraum von 2001–2005 erschienenen Veröffentlichungen zu geowissenschaftlichen Problemen des sächsischen Territoriums Schriften des Museums für Mineralogie und Geologie Dresden Ellen Kühne · Klaus Thalheim Ellen Kühne · KlausThalheim Nr. 15 – 2007 4 Die geologische Literatur über Sachsen 2001 – 2005 1 Titelbild J. C. Freiesleben: Magazin für die Oryktographie von Sachsen. Ein Beytrag zur Mineralogischen Kenntniß dieses Landes und zur Geschichte seiner Mineralien. Freyberg, 1828. Titelblatt Autoren Dipl.-Bibl. (FH) Ellen Kühne Prof. Dr. Klaus Thalheim Staatliche Naturhistorische Sammlungen Dresden Museum für Mineralogie und Geologie Königsbrücker Landstraße 159 D-01109 Dresden Impressum Herausgeber Staatliche Naturhistorische Sammlungen Dresden Museum für Mineralogie und Geologie Schriftleiter Dr. Jan-Michael Lange Vertrieb Staatliche Naturhistorische Sammlungen Dresden Naturhistorische Zentralbibliothek Abteilung Geowissenschaften Königsbrücker Landstraße 159 D-01109 Dresden Fon: +49-351 8926-268 Fax: +49-351 8926-404 E-mail: [email protected] Web: www.snsd.de Redaktionsschluss 31. Dezember 2006 Redaktion Daniela Erler, Martin Kaden, Ellen Kühne, Johanna Seibt, Klaus Thalheim Layout Markward Fischer Druckerei Druckhaus Dresden GmbH ISBN 978-3-910006-35-5 2 Ellen Kühne · Klaus Thalheim Die geologische Literatur über Sachsen 2001 – 2005 Bibliographie der im Zeitraum von 2001 – 2005 erschienenen Veröffentlichungen zu geowissenschaftlichen Problemen des sächsischen Territoriums Schriften des Museums für Mineralogie und Geologie Dresden Nr. 15 – 2007 3 4 Die geologische Literatur über Sachsen · 2001 – 2005 Vorwort Mit dieser Publikation in den Schriften des Unser Dank geht an zahlreiche Fachkollegen, Museums für Mineralogie und Geologie die mit Literaturhinweisen zu dieser Biblio- Dres den wird die 11.