(Solanum Tuberosum L.) MICROTUBER PRODUCTION
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From the Ground up the First Fifty Years of Mccain Foods
CHAPTER TITLE i From the Ground up the FirSt FiFty yearS oF mcCain FoodS daniel StoFFman In collaboratI on wI th t ony van l eersum ii FROM THE GROUND UP CHAPTER TITLE iii ContentS Produced on the occasion of its 50th anniversary Copyright © McCain Foods Limited 2007 Foreword by Wallace McCain / x by All rights reserved. No part of this book, including images, illustrations, photographs, mcCain FoodS limited logos, text, etc. may be reproduced, modified, copied or transmitted in any form or used BCE Place for commercial purposes without the prior written permission of McCain Foods Limited, Preface by Janice Wismer / xii 181 Bay Street, Suite 3600 or, in the case of reprographic copying, a license from Access Copyright, the Canadian Toronto, Ontario, Canada Copyright Licensing Agency, One Yonge Street, Suite 1900, Toronto, Ontario, M6B 3A9. M5J 2T3 Chapter One the beGinninG / 1 www.mccain.com 416-955-1700 LIBRARY AND ARCHIVES CANADA CATALOGUING IN PUBLICATION Stoffman, Daniel Chapter Two CroSSinG the atlantiC / 39 From the ground up : the first fifty years of McCain Foods / Daniel Stoffman For copies of this book, please contact: in collaboration with Tony van Leersum. McCain Foods Limited, Chapter Three aCroSS the Channel / 69 Director, Communications, Includes index. at [email protected] ISBN: 978-0-9783720-0-2 Chapter Four down under / 103 or at the address above 1. McCain Foods Limited – History. 2. McCain, Wallace, 1930– . 3. McCain, H. Harrison, 1927–2004. I. Van Leersum, Tony, 1935– . II. McCain Foods Limited Chapter Five the home Front / 125 This book was printed on paper containing III. -

Potato - Wikipedia, the Free Encyclopedia
Potato - Wikipedia, the free encyclopedia Log in / create account Article Talk Read View source View history Our updated Terms of Use will become effective on May 25, 2012. Find out more. Main page Potato Contents From Wikipedia, the free encyclopedia Featured content Current events "Irish potato" redirects here. For the confectionery, see Irish potato candy. Random article For other uses, see Potato (disambiguation). Donate to Wikipedia The potato is a starchy, tuberous crop from the perennial Solanum tuberosum Interaction of the Solanaceae family (also known as the nightshades). The word potato may Potato Help refer to the plant itself as well as the edible tuber. In the region of the Andes, About Wikipedia there are some other closely related cultivated potato species. Potatoes were Community portal first introduced outside the Andes region four centuries ago, and have become Recent changes an integral part of much of the world's cuisine. It is the world's fourth-largest Contact Wikipedia food crop, following rice, wheat and maize.[1] Long-term storage of potatoes Toolbox requires specialised care in cold warehouses.[2] Print/export Wild potato species occur throughout the Americas, from the United States to [3] Uruguay. The potato was originally believed to have been domesticated Potato cultivars appear in a huge variety of [4] Languages independently in multiple locations, but later genetic testing of the wide variety colors, shapes, and sizes Afrikaans of cultivars and wild species proved a single origin for potatoes in the area -
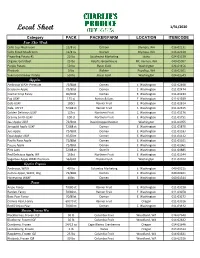
Local Sheet 1/31/2020
Local Sheet 1/31/2020 Category PACK PACKER/FARM LOCATION ITEMCODE New This Week Cello Cup Mushroom 12/8 oz Ostrom Olympia, WA 016-02131 Cello Sliced Mushroom 12/8 oz Ostrom Olympia, WA 016-02130 Fingerling Potato #1 20 lbs Southwind Marketing Idaho 024-01539 Organic Gold Beet 25 lbs Ralph's Greenhouse Mt. Vernon, WA 040-05097 Purple Potato 50 lbs Basin Gold Washington 024-01456 Rhubarb 5 lbs Richter Puyallup, WA 016-02301 Yukon Gold Baker Potato 50 lbs Basin Gold Washington 024-01549 Apples Ambrosia WAXF Premium 72/88ct Domex E. Washington 011-02468 Braeburn Apple 72/80ct Domex E. Washington 011-02474 Cosmic Crisp Fancy 40/60ct Domex E. Washington 011-01234 Fuji USXF 125 ct Borton & Sons E. Washington 011-01939 Gala USXF 100ct Rainier Fruit E. Washington 011-02614 Gala, USFCY 72/88 ct Rainier Fruit E. Washington 011-02615 Golden Delicious USXF 125ct Northern Fruit E. Washington 011-01176 Granny Smith USXF 100 ct Northern Fruit E. Washington 011-01751 Jazz Apple USXF 72/80ct David Doppenheimer Washington 011-01955 Jonagold Apple USXF 72/88 ct Domex E. Washington 011-01870 Juci Apple 72/88ct Domex E. Washington 011-01183 Opal Apple USXF 45/60ct Domex E. Washington 011-01112 Pacific Rose Apple 72/88ct Domex E. Washington 011-01101 Pazzaz Apple 72/88ct Domex E. Washington 011-01061 Pink Lady 72/88 ct Stemilt E. Washington 011-01885 Red Delicious Apple, WF 163ct Northern Fruit E. Washington 011-01544 Sugarbee Apple WAXF Premium 56/64ct Chelan Fresh Washington 011-02012 Apples Organic Ambrosia, WAXF 40 lbs Columbia Marketing E. -

Potato Glossary
A Potato Glossary A Potato Glossary by Richard E. Tucker Last revised 15 Sep 2016 Copyright © 2016 by Richard E. Tucker Introduction This glossary has been prepared as a companion to A Potato Chronology. In that work, a self-imposed requirement to limit each entry to a single line forced the use of technical phrases, scientific words, jargon and terminology that may be unfamiliar to many, even to those in the potato business. It is hoped that this glossary will aid those using that chronology, and it is hoped that it may become a useful reference for anyone interested in learning more about potatoes, farming and gardening. There was a time, a century or more ago, when nearly everyone was familiar with farming life, the raising of potatoes in particular and the lingo of farming in general. They were farmers themselves, they had relatives who farmed, they knew someone who was a farmer, or they worked on a nearby farm during their youth. Then, nearly everyone grew potatoes in their gardens and sold the extra. But that was a long ago time. Now the general population is now separated from the farm by several generations. Only about 2 % of the US population lives on a farm and only a tiny few more even know anyone who lives on a farm. Words and phrases used by farmers in general and potato growers in particular are now unfamiliar to most Americans. Additionally, farming has become an increasingly complex and technical endeavor. Research on the cutting edge of science is leading to new production techniques, new handling practices, new varieties, new understanding of plant physiology, soil and pest ecology, and other advances too numerous to mention. -

Plant Availability 04/27/2020 ACER GRISEUM #7 $145.99 2 ACER PAL
Whitney's Farm 1775 South State Road ~ Cheshire MA 413-442-4749 Plant Availability 04/27/2020 ACER GRISEUM #7 $145.99 2 ACER PAL. DIS. `INABA SHIDARE` #3 $104.99 10 ACER PAL. DIS. `INABA SHIDARE` #5 $183.99 5 ACER PAL. DIS. `TAMUKEYAMA` #3 $104.99 10 ACER PAL. DIS. `TAMUKEYAMA` #5 $183.99 5 ACER PAL. DIS. `VIRIDIS` #3 $104.99 5 ACER PALMATUM `BLOODGOOD` #3 $104.99 10 ACER PALMATUM `BLOODGOOD` #5 $183.99 5 ACER PALMATUM `EMPEROR I` #5 $183.99 2 ACER PALMATUM `EMPEROR I` #3 $104.99 10 ACER PALMATUM `ORANGE DREAM` #3 $104.99 5 ACER PALMATUM `PEACHES & CREAM` #3 $104.99 10 ACER RUBRUM `RED SUNSET` #15 $217.99 6 ACER RUBRUM `REDPOINTE` #7 $145.99 6 ACER SACCHARUM `GREEN MOUNTAIN` #15 $232.99 6 ACER SACCHARUM `SUPER SWEET` #7 $150.99 2 ACTINIDIA ARGUTA `ISSAI` #2 $40.99 5 AGASTACHE X `BLUE FORTUNE` #2 $17.99 12 AZALEA `ARCTIC ROSE` #3 $41.99 24 AZALEA `BIXBY` #2 $31.99 8 AZALEA `DELAWARE VALLEY WHITE` #3 $41.99 8 AZALEA `GIRARD`S FUCHSIA` #3 $41.99 8 AZALEA `GIRARD`S PLEASANT WHITE` #3 $41.99 8 AZALEA `KAREN` #3 $41.99 8 AZALEA `LEMON LIGHTS` #3 $58.99 8 AZALEA `MANDARIN LIGHTS` #3 $58.99 16 AZALEA `ROSEBUD` #3 $41.99 8 AZALEA `STEWARTSTONIAN` #3 $41.99 8 AZALEA ARBORESCENS #2 $41.99 8 AZALEA VISCOSUM `PINK AND SWEET` #3 $58.99 8 BETULA JACQUEMONTII - CLUMP #15 $190.99 2 BETULA NIGRA `HERITAGE` - CLUMP #15 $190.99 4 BETULA PAPYRIFERA - CLUMP #7 $126.99 2 BETULA PLATYPHYLLA `WHITESPIRE` - CLUMP #15 $190.99 2 BUDDLEIA `LO & BEHOLD PINK MICCHIP` #3 $43.99 8 BUDDLEIA DAVIDII `BLACK KNIGHT` #3 $38.99 8 BUDDLEIA DAVIDII `NANHO BLUE` #3 $38.99 8 BUDDLEIA PUGSTER `BLUE` #3 $43.99 8 BUDDLEIA PUGSTER `PINK` #3 $43.99 8 BUDDLEIA PUGSTER `WHITE` #3 $43.99 8 BUDDLEIA X `MISS MOLLY` #3 $43.99 8 BUDDLEIA X `MISS RUBY` #3 $43.99 8 BUDDLEIA X `MISS VIOLET` #3 $43.99 8 BUXUS MICRO. -

Hugo Campos · Oscar Ortiz Editors the Potato Crop Its Agricultural, Nutritional and Social Contribution to Humankind the Potato Crop Hugo Campos • Oscar Ortiz Editors
Hugo Campos · Oscar Ortiz Editors The Potato Crop Its Agricultural, Nutritional and Social Contribution to Humankind The Potato Crop Hugo Campos • Oscar Ortiz Editors The Potato Crop Its Agricultural, Nutritional and Social Contribution to Humankind Editors Hugo Campos Oscar Ortiz International Potato Center International Potato Center Lima, Peru Lima, Peru ISBN 978-3-030-28682-8 ISBN 978-3-030-28683-5 (eBook) https://doi.org/10.1007/978-3-030-28683-5 This book is an open access publication. © The Editor(s) (if applicable) and The Author(s) 2020 Open Access This book is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this book are included in the book’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the book's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. -

Commercial Potato Production in North America
Commercial Potato Production in North America The Potato Association of America Handbook Second Revision of American Potato Journal Supplement Volume 57 and USDA Handbook 267 by the Extension Section of The Potato Association of America Commercial Potato Production in North America Editors William H. Bohl, University of Idaho Steven B. Johnson, University of Maine Contributing Authors to the 2010 Revision Stephen Belyea Maine Dept. of Agric., Food & Rural Resources Chuck Brown USDA-ARS, Washington Alvin Bushway University of Maine Alvin Bussan University of Wisconsin-Madison Rob Davidson Colorado State University Joe Guenthner University of Idaho Bryan Hopkins Brigham Young University Pamela J. S. Hutchinson University of Idaho Steven B. Johnson University of Maine Gale Kleinkopf University of Idaho, Retired Jeff Miller Miller Research (formerly University of Idaho) Nora Olsen University of Idaho Paul Patterson University of Idaho Mark Pavek Washington State University Duane Preston University of Minnesota, Retired Edward Radcliffe University of Minnesota, Retired Carl Rosen University of Minnesota Peter Sexton South Dakota State University Clinton Shock Oregon State University Joseph Sieczka Cornell University, Retired David Spooner USDA-ARS, Wisconsin Jeffrey Stark University of Idaho Walt Stevenson University of Wisconsin, Retired Asunta Thompson North Dakota State University Mike Thornton University of Idaho Robert Thornton Washington State University, Retired October 26, 2010 Commercial Potato Production in North America A Brief History of this Handbook and Use of the Information The current publication, Commercial Potato Production in North America was originally published in July 1964 by the USDA Agricultural Research Service, Agriculture Handbook No. 267, with the title, Commercial Potato Production, authored by August E. -

424 Executive Council 16 November 2004
424 EXECUTIVE COUNCIL __________________________ 16 NOVEMBER 2004 EC2004-665 AGRICULTURAL INSURANCE ACT GENERAL REGULATIONS Pursuant to section 16 of the Agricultural Insurance Act R.S.P.E.I. 1988, Cap. A-8.2, the Board of the Prince Edward Island Agricultural Insurance Corporation, with the approval of the Lieutenant Governor in Council, made the following regulations: 1. In these regulations Definitions (a) “acreage” means the land area planted in a crop or variety, acreage expressed in acres or hectares, and stated on the application form for a crop year; (b) “Act” means the Agricultural Insurance Act R.S.P.E.I. 1988, Act Cap. A-8.2; (c) “annual index” for an insured in respect of a crop group, means annual index the ratio between (i) the insured’s production to count in a crop year for the crop group, and (ii) the average production to count for the province as a whole for the crop group and for that crop year; (d) “Appeal Board” means the Appeal Board established under Appeal Board section 14 of the Act; (e) “benchmark yield” is the simple average of the preceding five benchmark yield, years’ provincial weighted average yield per acre for a crop or variety or is an average calculated by such means as is acceptable to the Board; (f) “Board” means the board of directors of the Corporation; Board (g) “Corporation” means the Prince Edward Island Agricultural Corporation Insurance Corporation; (h) “coverage level” means the percentage of the probable yield of a coverage level crop in any risk area or in any farm enterprise that is insured -
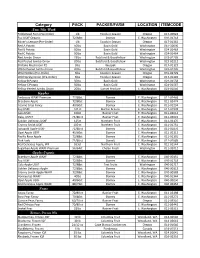
Category PACK PACKER/FARM LOCATION ITEMCODE New This Week Apples Pears
Category Strawberry, Frozen IQF 30 lb Columbia Fruit Woodland, WA 014-02643 Mushrooms Black Trumpet Mushrooms Pre-Order 5lbs Foods in Season Oregon 016-02020 Cello Cup Mushroom 12/8 oz Ostrom Olympia, WA 016-02131 Cello Sliced Mushroom 12/8 oz Ostrom Olympia, WA 016-02130 Crimini 5 lbs Ostrom Olympia, WA 016-02069 Crimini Sliced Cup 12/8oz Ostrom Olympia, WA 016-02052 Crimini Whole Cup 12/8oz Ostrom Olympia,WA 016-02051 Crimini Whole 8oz Ostrom Olympia,WA 031-01546 Hedgehog Mushrooms 5lbs Foods in Season Washington 016-02090 Oyster 5 lbs White Forest Mushrooms Turner, OR 017-01109 Portabello, Chunks 5 lbs Ostrom Olympia, WA 016-02082 Shiitake 5 lbs Wespak Oregon 017-01122 Shiitake Mushroom #2 5lbs Wespak Oregon 017-01123 Sliced Portabello Cap 8/8 oz Ostrom Olympia, WA 016-06228 Thick Sliced #2 10 lbs Ostrom Olympia, WA 016-02126 Thin Sliced #1 5 lbs Ostrom Olympia, WA 016-02125 White Large 10 lbs Ostrom Olympia, WA 016-02110 White Large 5 lbs Ostrom Olympia, WA 016-02112 Yellow Foot Mushrooms 5lbs Foods in Season Oregon 016-02028 Mushrooms Organic Oyster 5 lbs White Forest Mushrooms Oregon 040-04302 Shiitake 5 lbs Wespak Oregon 040-04421 Onions Jumbo Onions 50 lbs Sunset Produce E. Washington 023-01008 Red Onion 50lbs Botsford & Goodfellow Washington 023-01207 Red Jumbo Onion 25lbs Botsford & Goodfellow Washington 023-01205 Red Peeled Jumbo Onion 20lbs Botsford & Goodfellow Washington 023-01212 Super Colossal Yellow Onion 50 lbs Sunset Produce Washington 023-01090 Washington Sweet 40 lbs Sunset Produce E. Washington 023-01209 White Jumbo Onion 50lbs Botsford & Goodfellow Washington 023-01218 White Peeled Jumbo Onion 20lbs Botsford & Goodfellow Washington 023-01226 Wild Spring Onion (Pre-Order) 3lbs Foods in Season Oregon 017-01402 Yellow Colossal Onion 50lbs Botsford & Goodfellow Washington 023-01012 Yellow Peeled Jumbo Onion 20lbs Sunset Produce E. -

Hugo Campos · Oscar Ortiz Editors the Potato Crop Its Agricultural, Nutritional and Social Contribution to Humankind the Potato Crop Hugo Campos • Oscar Ortiz Editors
Hugo Campos · Oscar Ortiz Editors The Potato Crop Its Agricultural, Nutritional and Social Contribution to Humankind The Potato Crop Hugo Campos • Oscar Ortiz Editors The Potato Crop Its Agricultural, Nutritional and Social Contribution to Humankind Editors Hugo Campos Oscar Ortiz International Potato Center International Potato Center Lima, Peru Lima, Peru ISBN 978-3-030-28682-8 ISBN 978-3-030-28683-5 (eBook) https://doi.org/10.1007/978-3-030-28683-5 This book is an open access publication. © The Editor(s) (if applicable) and The Author(s) 2020 Open Access This book is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this book are included in the book’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the book's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. -

Commercial Potato Production in North America Handbook
Commercial Potato Production in North America The Potato Association of America Handbook Second Revision of American Potato Journal Supplement Volume 57 and USDA Handbook 267 by the Extension Section of The Potato Association of America Commercial Potato Production in North America Editors William H. Bohl, University of Idaho Steven B. Johnson, University of Maine Contributing Authors to the 2010 Revision Stephen Belyea Maine Dept. of Agric., Food & Rural Resources Chuck Brown USDA‐ARS, Washington Alvin Bushway University of Maine Alvin Bussan University of Minnesota‐Madison Rob Davidson Colorado State University Joe Guenthner University of Idaho Bryan Hopkins Brigham Young University Pamela J. S. Hutchinson University of Idaho Steven B. Johnson University of Maine Gale Kleinkopf University of Idaho, Retired Jeff Miller Miller Research (formerly University of Idaho) Nora Olsen University of Idaho Paul Patterson University of Idaho Mark Pavek Washington State University Duane Preston University of Minnesota, Retired Edward Radcliffe University of Minnesota, Retired Carl Rosen University of Minnesota Peter Sexton South Dakota State University Clinton Shock Oregon State University Joseph Sieczka Cornell University, Retired David Spooner USDA‐ARS, Wisconsin Jeffrey Stark University of Idaho Walt Stevenson University of Wisconsin, Retired Asunta Thompson North Dakota State University Mike Thornton University of Idaho Robert Thornton Washington State University, Retired October 26, 2010 Commercial Potato Production in North America A Brief History of this Handbook and Use of the Information The current publication, Commercial Potato Production in North America was originally published in July 1964 by the USDA Agricultural Research Service, Agriculture Handbook No. 267, with the title, Commercial Potato Production, authored by August E. -
2019 Crop Director Y
WisconsinCERTIFIED SEED POTATOES WISCONSIN CERTIFIED SEED POTATOES WISCONSIN CERTIFIED SEED POTATOES www.potatoseed.org WISCONSIN CERTIFIED SEED POTATOES 2019 CROP DIRECTORY HAVING THE HIGHEST STRENGTH-TO-WEIGHT RATIO IN THE INDUSTRY ISN’T AN ACCIDENT. EXPECT PERFORMANCE AND DURABILITY LIKE NO OTHER. It starts with high tensile steel that creates an unmatched combination of high strength and lighter weight. Then Reinke engineers create innovations like the single leg tower, one of the widest tower bases in the industry and up to 6,000 pounds of overall system weight savings. In the end, a Reinke system will out last (with one of the longest structural warranties around) and outperform while saving you time and energy in the long run. 1500 Post Road | Plover WI 54467 | (715) 344-4747 2022 W. 2nd Avenue | Bloomer, WI 54724 | (715) 568-4600 www.robertsirrigation.com Sand County Equipment Stocks the INDUSTRY’S LARGEST INVENTORY of New and Used Equipment! HARRISTON MAYO PILER & MAYO SURGE HOPPER CLODHOPPERServicing ALL MAKES of Equipment CONVEYOR LINE Introducing the all new Harriston Clamp Planter! WIL-RICH harristonmayo.com Farm King HARRISTON MAYO PILER & MAYO SURGE HOPPER CLODHOPPER CONVEYOR LINE Introducing the all new Harriston Clamp Planter! harristonmayo.com R AVEN Shop: (715) 335-6652 Cell: (715) 498-6651 8364 Monica Road, PO Box 228 Bancroft, WI 54921 E-mail: [email protected] OWNER: PAUL CIESLEWICZ www.sandcountyequipment.com SINGLE PAGE OPPOSITE INSIDE FRONT COVER WISCONSIN CERTIFIED SEED POTATOES WISCONSIN CERTIFIED SEED POTATOES The program maintains variety trueness to type; selecting and testing clones, rogueing of weak, genetic variants, and diseased plants to continue to develop and maintain germplasm of your WISCONSIN favorite varieties at our laboratory.