Bsc Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plant Physiology and Plant Development
Plant Physiology and Plant Development PTS 351 B.Sc. B.Ed. Semester – VI Course Instructor Dr. Gautam Kumar Dr. Gautam Kr. Dept. of Life Sc. 1 Carbon Assimilation Light absorption and energy conversion Carbon dioxide uptake and assimilation Calvin Cycle (C3 Pathway) Hatch-Slack pathway (C4 Pathway) Photorespiration (C2 Pathway)/Glycolate metabolism Dr.Gautam Kr. Dept. of Life Sc. 2 • Visible light is electromagnetic radiation of wavelengths 400 to 700 nm, a small part of the electromagnetic spectrum ranging from violet to red. • The energy of a single photon (a quantum of light) is greater at the violet end of the spectrum than at the red end; shorter wavelength (and higher frequency) corresponds to higher energy. Dr.Gautam Kr. Dept. of Life Sc. 3 Solar energy as the ultimate source of all biological energy Photosynthetic organisms use the energy of sunlight to manufacture glucose and other organic products, which heterotrophic cells use as energy and carbon sources. The light reactions of photosynthesis generate energy rich NADPH and ATP at the expense of solar energy. These products are used in the carbon assimilation reactions, which occur in light or darkness, to reduce CO2 to form trioses and more complex compounds (such as glucose) derived from trioses. Dr.Gautam Kr. Dept. of Life Sc. 4 Absorption of visible light by Photo-pigments Plants are green because their pigments absorb light from the red and blue regions of the spectrum, leaving primarily green light to be reflected or transmitted Absorption spectra of the pigments with the spectrum of sunlight reaching the earth’s surface Dr.Gautam Kr. -

Cellular Respiration How Is Energy Transferred and Transformed in Living Systems? Why ?
Cellular Respiration How is energy transferred and transformed in living systems? Why ? Living organisms display the property of metabolism, which is a general term to describe the processes carried out to acquire and use energy. We know that people need to eat, and in our foods are various kinds of nutrients that our cells use. One large group of nutrients in our foods is carbohydrates, which supply our cells with glucose (C6H12O6). So the question is: How does the food we chew and swallow fuel our cells? Model 1 – Glycolysis Cell membrane NAD NADH ADP ATP ADP NAD ! = pyruvic acid (3 C) ATP NADH = glucose (6 C) = mitochondrion = nucleus NUCLEUS 1. Refer to Model 1. a. What is represented by the hexagon? GLUCOSE b. How many carbon atoms (C) are in one molecule of glucose? SIX 2. Refer to Model 1. a. What is represented by the triangles? PYRUVIC ACID b. How many carbon atoms (C) are in one molecule of pyruvic acid? THREE 3. In the process of glycolysis, what happens to glucose after it crosses the cell membrane into the cytoplasm of the cell? GLUCOSE IS BROKEN DOWN INTO PYRUVIC ACID (2) Cellular 1 Respiration Read This! Glycolysis occurs in the cytoplasm of cells and does not require the presence of oxygen. Therefore, the process is anaerobic. It is the first step used by cells to extract energy from glucose in the form of ATP. ATP can be directly used by cells. 4. Thinking about the number of carbon atoms in glucose and in pyruvic acid, explain why there is one molecule of glucose on the left side of the arrow and two molecules of pyruvic acid on the right side of the arrow. -

1 Cellular Respiration: Harvesting Chemical Energy Introduction
Cellular Respiration: Harvesting Chemical Energy Chapter 9 • Objectives • Define oxidation and reduction, and, in general terms, explain how redox reactions are involved in energy exchanges. • Name the three stages of cellular respiration and state the region of the eukaryotic cell where each stage occurs. • In general terms, explain the role of the electron transport chain in cellular respiration. • Explain where and how the respiratory electron transport chain creates a proton gradient. • Distinguish between fermentation and anaerobic respiration 2 Introduction • Harvesting chemical energy forms part of a cycle involving mitochondria and chloroplasts 3 1 Catabolic Pathways and Production of ATP • Slow burning of food generates ATP – the breakdown of organic molecules is exergonic • In the absence of O 2 food molecules are “fermented” – sugars are partially degraded 5 • Cellular respiration is the most prevalent and efficient catabolic pathway – consumes oxygen and organic molecules such as glucose and yields ATP 6 2 • To keep working cells must regenerate ATP – cell respiration stores energy in ATP molecules • overall equation: –C6H12 O6 + 6O 2 → 6CO 2 + 6H 2O + energy • efficiency ~40% compared with car ~25% • Energy is used for body maintenance and voluntary activity – average human needs ~2200kcal/day 7 Redox Reactions: Oxidation and Reduction • Catabolic pathways yield energy due to the transfer of electrons • Paired endergonic-exergonic reactions are known as redox (reduction-oxidation) reactions – redox reactions transfer electrons -

ATP and Respiration
ATP and Respiration Wednesday, January 24, 2018 Energy “the ability to do work” is needed for: 1. Metabolism All reactions that take place in living organisms involve energy 2. Movement Within (circulation) and whole organism (locomotion) 3. Active Transport Movement of ions and molecules across a plasma membrane against a concentration gradient 4. Maintenance, repair and division Of organelles and cells 5. Production of substances Eg. Hormones and enzymes 6. Maintenance of body temperature For Endothermic organisms Where does this energy come from? Ultimately, all energy comes from the SUN as light energy (energy can never be lost or created, only converted) Green plants and algae convert light energy into chemical energy, in the form of organic molecules such as glucose, via PHOTOSYNTHESIS Consumers then ingest these organic molecules as food and use them for RESPIRATION. Producers use their ‘own’ organic molecules for respiration. HOWEVER… Cellular Respiration in mitochondria is NOT the energy source. Respiration instead converts organic molecules into ATP which IS the source of energy in organisms. Hence we have always told you that mitochondria are the site of aerobic respiration or site of production of ATP, not the source of energy in cells. Adenosine TriPhosphate ADENINE (same as the purine base) PHOSPHATE RIBOSE (a pentose sugar) Garage Chimney smoke House The three phosphate groups are the key to how ATP stores energy – they are only connected by weak, unstable bonds which are easily broken, releasing a large amount of energy. H2O ATP Water ADP Pi Inorganic ENERGY phosphate What type of reaction is this? HYDROLYSIS – addition of water This reaction is actually reversible… ATP is hydrolysed to provide energy for reactions that require it… HYDROLYSIS H2O CONDENSATION ATP Water ADP Pi Inorganic ENERGY phosphate ATP is reformed from ADP + Pi via a condensation reaction during reactions that generate energy. -

Advanced Cell Biology. Lecture 7
Advanced Cell Biology. Lecture 7 Advanced Cell Biology. Lecture 7 Alexey Shipunov Minot State University January 27, 2012 Advanced Cell Biology. Lecture 7 Outline Questions and answers Nucleic acids Structure Other nucleic acids Macromolecules in cells Cells and energy Advanced Cell Biology. Lecture 7 Outline Questions and answers Nucleic acids Structure Other nucleic acids Macromolecules in cells Cells and energy Advanced Cell Biology. Lecture 7 Outline Questions and answers Nucleic acids Structure Other nucleic acids Macromolecules in cells Cells and energy Advanced Cell Biology. Lecture 7 Outline Questions and answers Nucleic acids Structure Other nucleic acids Macromolecules in cells Cells and energy I TAACCTTCG I DNA Advanced Cell Biology. Lecture 7 Questions and answers Previous final question: the answer Write a sequence complementary to ATTGGAAGC Is it from DNA or RNA? Advanced Cell Biology. Lecture 7 Questions and answers Previous final question: the answer Write a sequence complementary to ATTGGAAGC Is it from DNA or RNA? I TAACCTTCG I DNA Advanced Cell Biology. Lecture 7 Nucleic acids Structure Nucleic acids Structure Advanced Cell Biology. Lecture 7 Nucleic acids Structure Hydrogen bonds in complementary strands Advanced Cell Biology. Lecture 7 Nucleic acids Structure Double helix I DNA form helical structure where phosphate and sugar form “envelope” and bases form a “core” I Two grooves: major and minor Advanced Cell Biology. Lecture 7 Nucleic acids Structure DNA double helix Advanced Cell Biology. Lecture 7 Nucleic acids Structure DNA double helix from top Advanced Cell Biology. Lecture 7 Nucleic acids Structure Sequences, ends, abbreviations I Since nucleotides are complementary, it is usually only one strand listed I Each strand has 3’ (–OH) and 5’ ends (phosphate) Advanced Cell Biology. -

How Cells Harvest Chemical Energy
How Cells Harvest Chemical Energy Global Athlete Outreach Program US CytoThesis Systems Medicine Center www.CytoThesis.US US OncoTherapy Systems BioMedicine Group CytoThesis Bioengineering Research Group General Biology – Dept Mathematics Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings How is a Marathoner Different from a Sprinter ? • Long-distance runners have many slow muscle fibers in their muscles – Slow muscle fibers break down glucose for ATP production aerobically using oxygen – These muscle cells can sustain repeated, long contractions Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings • Sprinters have more fast muscle fibers – Fast fibers make ATP without oxygen— anaerobically – They can contract quickly and supply energy for short bursts of intense activity Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings • The dark meat of a cooked turkey is an example of slow fiber muscle – Leg muscles support sustained activity – The white meat consists of fast fibers (less myoglobin) – Wing muscles allow for quick bursts of flight dark meat white meat Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings INTRODUCTION TO CELLULAR RESPIRATION • Nearly all the cells in our body break down sugars for ATP production • Most cells of most organisms harvest energy aerobically, like slow muscle fibers – The aerobic harvesting of energy from sugar is called cellular respiration – Cellular respiration yields CO2, H2O, and a large amount of ATP Copyright © 2003 Pearson Education, Inc. publishing as Benjamin Cummings 6.1 Breathing supplies oxygen to our cells and removes carbon dioxide • Breathing and cellular respiration are closely related BREATHING O2 CO2 Lungs CO2 Bloodstream O2 Muscle cells carrying out CELLULAR RESPIRATION Sugar + O2 ATP + CO2 + H2O Figure 6.1 Copyright © 2003 Pearson Education, Inc. -

Carbohydrate: Constructing the Carbon-Neutral Carbohydrate Economy
Energies 2011, 4, 254-275; doi:10.3390/en4010254 OPEN ACCESS energies ISSN 1996-1073 www.mdpi.com/journal/energies Review Renewable Hydrogen Carrier — Carbohydrate: Constructing the Carbon-Neutral Carbohydrate Economy Y.-H. Percival Zhang 1,2,3,4,* and Jonathan R. Mielenz 3,5 1 Biological Systems Engineering Department, 210-A Seitz Hall, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061, USA 2 Institute for Critical Technology and Applied Sciences (ICTAS) Virginia Polytechnic Institute and State University, Blacksburg, VA 24061, USA 3 DOE BioEnergy Science Center (BESC), Oak Ridge, TN 37831, USA; E-Mail: [email protected] 4 Gate Fuels Inc. 3107 Alice Drive, Blacksburg, VA 24060, USA 5 Biosciences Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-540-231-7414; Fax: +1-540-231-3199. Received: 15 December 2010; in revised form: 8 January 2011 / Accepted: 28 January 2011 / Published: 30 January 2011 Abstract: The hydrogen economy presents an appealing energy future but its implementation must solve numerous problems ranging from low-cost sustainable production, high-density storage, costly infrastructure, to eliminating safety concern. The use of renewable carbohydrate as a high-density hydrogen carrier and energy source for hydrogen production is possible due to emerging cell-free synthetic biology technology—cell-free synthetic pathway biotransformation (SyPaB). Assembly of numerous enzymes and co-enzymes in vitro can create complicated set of biological reactions or pathways that microorganisms or catalysts cannot complete, for example, C6H10O5 (aq) + 7 H2O (l) 12 H2 (g) + 6 CO2 (g) (PLoS One 2007, 2:e456). -
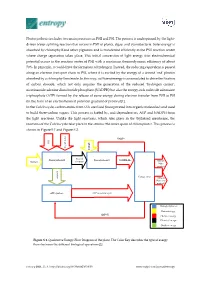
Photosynthesis Includes Two Main Processes As PSII and PSI
Photosynthesis includes two main processes as PSII and PSI. The process is underpinned by the light- driven water splitting reaction that occurs in PSII of plants, algae, and cyanobacteria. Solar energy is absorbed by chlorophyll and other pigments and is transferred efficiently to the PSII reaction center where charge separation takes place. This initial conversion of light energy into electrochemical potential occurs in the reaction center of PSII with a maximum thermodynamic efficiency of about 70%. In principle, it could drive the formation of hydrogen. Instead, the reducing equivalent is passed along an electron transport chain to PSI, where it is excited by the energy of a second ‘red’ photon absorbed by a chlorophyll molecule. In this way, sufficient energy is accumulated to drive the fixation of carbon dioxide, which not only requires the generation of the reduced ‘hydrogen carrier’, nicotinamide adenine dinucleotide phosphate (NADPH) but also the energy-rich molecule adenosine triphosphate (ATP) formed by the release of some energy during electron transfer from PSII to PSI (in the form of an electrochemical potential gradient of protons)[1]. In the Calvin cycle, carbon atoms from CO2 are fixed (incorporated into organic molecules) and used to build three-carbon sugars. This process is fueled by, and dependent on, ATP and NADPH from the light reactions. Unlike the light reactions, which take place in the thylakoid membrane, the reactions of the Calvin cycle take place in the stroma (the inner space of chloroplasts). This process is shown in Figure S 1 and Figure S 2. NADP+ CO2 Liquid water Excited Photosynthesis II Photosynthesis I NADPH+H+ Sunlight electrons Glucose Main product protons Calvine cycle Oxygen Waste(co- product) ATP synthase Biological process Photon Exergy ADP+Pi Chemical exergy Electrical exergy Gradient exergy Figure S 1. -
Photosynthesis and Cellular Respiration
Biology – unit 3 examiners report; photosynthesis and cellular respiration We will be starting at 4:30pm. In the meantime, please check the following: • your computer speakers are on and volume is up, and your microphone is muted • click the chat icon to open the chat feed – use this to ask us questions • you have the accompanying Masterclass documents in front of you for reference If you are having any issues, please send us a message in the chat feed Exam Pitfalls • Some Common Pitfalls in Exam answers. 1. Not answering the question a. Always read the question in totality. Then go back and read it again to check that you have understood what is been asked. b. You interpret the question differently and you start to answer it, but you are off topic. Why? Some students haven’t had time to revise correctly, they have a set of pre-prepared answers that they fall back on or they have panicked into thinking they have understood the question. 2. Not looking at the mark scheme or the space provided a. Both the mark scheme and the space provided (if there is one) will provide clues about how much the examiners are expecting to see. Dot points are acceptable. A 4 mark question is not going to be answered by a one word response! 3. Panicking a. Some students go blank when they see the exam and it’s not what they think it should be. Step back. Take a deep breath, count to ten and then look at the paper again. -

Biochemistry Generation of ATP
Paper : 04 Metabolism of carbohydrates Module :18 Generation of ATP Principal Investigator Dr.S.K.Khare, Professor IIT Delhi. Paper Coordinator Dr. Ramesh Kothari, Professor UGC-CAS Department of Biosciences Saurashtra University, Rajkot-5, Gujarat-INDIA Content Reviewer Dr. S. P. Singh, Professor UGC-CAS Department of Biosciences Saurashtra University, Rajkot-5, Gujarat-INDIA Dr. Ramesh Kothari, Professor Content Writer UGC-CAS Department of Biosciences Saurashtra University, Rajkot-5, Gujarat-INDIA 1 Metabolism of Carbohydrates Biochemistry Generation of ATP Description of Module Subject Name Biochemistry Paper Name 04 Metabolism of carbohydrates Module 18 Generation of ATP Name/Title 2 Metabolism of Carbohydrates Biochemistry Generation of ATP Bio chemical mechanisms of generating ATP In metabolisms, ATP is generated by two fundamental different biochemical mechanisms: 1. Substrate level phosphorylation ,and 2. Electron transport chain. Substrate level phosphorylation In substrate level phosphorylation, ATP is formed from ADP by transfer of a high energy phosphate group from an intermediate of a fueling pathway. The following reaction serve as an example As a consequence of the removal of a molecule of water, the low- energy ester linkage of phosphate in 2- phosphoglyceric acid is converted to the high energy enol linkage in phosphoenol pyruvic acid. 3 Metabolism of Carbohydrates Biochemistry Generation of ATP This high- energy linked phosphate can then then be transferred to ADP, the consequence of which is generation of a molecule of ATP. Generation of ATP by Electron Transport In a number of different mode of microbial metabolisms including respiration and photosynthesis, ATP is generated by transporting electron through the chain of carrier molecules with fixed orientation in a cell membrane. -

13 Cellular Respiration-S
Cellular Respiration How is energy transferred and transformed in living systems? Why? Living organisms display the property of metabolism, which is a general term to describe the processes carried out to acquire and use energy. We know that people need to eat, and in our foods are various kinds of nutrients that our cells use. One large group of nutrients in our foods is carbohydrates, which supply our cells with glucose (C6H12O6). So the question is: How does the food we chew and swallow fuel our cells? Model 1 – Glycolysis Cell membrane NAD NADH ADP ATP ! = pyruvic acid (3 C) NADH ADP NAD ATP = glucose (6 C) = mitochondrion = nucleus 1. Refer to Model 1. a. What is represented by the hexagon? b. How many carbon atoms (C) are in one molecule of glucose? 2. Refer to Model 1. a. What is represented by the triangles? b. How many carbon atoms (C) are in one molecule of pyruvic acid? 3. In the process of glycolysis, what happens to glucose after it crosses the cell membrane into the cytoplasm of the cell? Cellular Respiration 1 Read This! Glycolysis occurs in the cytoplasm of cells and does not require the presence of oxygen. Therefore, the process is anaerobic. It is the fi rst step used by cells to extract energy from glucose in the form of ATP. ATP can be directly used by cells. 4. Thinking about the number of carbon atoms in glucose and in pyruvic acid, explain why there is one molecule of glucose on the left side of the arrow and two molecules of pyruvic acid on the right side of the arrow. -

Chloroplast Fluorescence: Evidence for a Cyclic, Proton-Conducting Pathway in Oxygenic Photosynthesis (Photosystem II/Cyclic Proton Transport/Uncouplers) STEVEN W
Proc. Natl. Acad. Sci. USA Vol. 84, pp. 8424-8428, December 1987 Biophysics Protonophores induce plastoquinol oxidation and quench chloroplast fluorescence: Evidence for a cyclic, proton-conducting pathway in oxygenic photosynthesis (photosystem II/cyclic proton transport/uncouplers) STEVEN W. MCCAULEY*, ANASTASIOS MELIS, GEORGE M.-S. TANG, AND DANIEL 1. ARNON Division of Molecular Plant Biology, University of California, Berkeley, CA 94720 Contributed by Daniel 1. Arnon, August 7, 1987 ABSTRACT The photosynthetic apparatus converts light terminal acceptor, PQH2 accumulates; electrons then "back into chemical energy by a series of reactions that give rise to a up" and accumulate on a specialized PQ (QA). The accumu- coupled flow of electrons and protons that generate reducing lation of QA is monitored by a rise in variable fluorescence power and ATP, respectively. A key intermediate in these (Fv) (6). reactions is plastoquinone (PQ), the most abundant electron We have found that in the absence of a terminal acceptor, and proton (hydrogen) carrier in photosynthetic membranes two chemically diverse proton-conducting ionophores (thylakoids). PQ ultimately transfers electrons to a terminal (uncouplers), 2,6-di-t-butyl-4-(2',2'-dicyanovinyl)phenol (SF electron acceptor by way of the Rieske Fe-S center of the 6847) and carbonylcyanide p-trifluoromethoxyphenylhydra- cytochrome bfcomplex. In the absence of a terminal acceptor, zone (FCCP), induced oxidation of PQH2 and dramatically electrons accumulate in the PQ pool, which is reduced to lowered chloroplast fluorescence (signifying oxidation of plastoquinol (PQH2), and also on a specialized PQ, QA, which Q-). The two protonophores produced the same effects when is reduced to an unprotonated semiquinone anion (Q-).