Supplementary Material Automatic Parametrization of Non-Polar Implicit Solvent Models for the Blind Prediction of Solvation Free Energies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Provisional Peer-Reviewed Toxicity Values for Sulfolane (Casrn 126-33-0)
EPA/690/R-12/024F l Final 1-30-2012 Provisional Peer-Reviewed Toxicity Values for Sulfolane (CASRN 126-33-0) Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 AUTHORS, CONTRIBUTORS, AND REVIEWERS CHEMICAL MANAGER Dan D. Petersen, PhD, DABT National Center for Environmental Assessment, Cincinnati, OH DRAFT DOCUMENT PREPARED BY ICF International 9300 Lee Highway Fairfax, VA 22031 PRIMARY INTERNAL REVIEWERS Ghazi Dannan, PhD National Center for Environmental Assessment, Washington, DC Q. Jay Zhao, PhD, MPH, DABT National Center for Environmental Assessment, Cincinnati, OH This document was externally peer reviewed under contract to Eastern Research Group, Inc. 110 Hartwell Avenue Lexington, MA 02421-3136 Questions regarding the contents of this document may be directed to the U.S. EPA Office of Research and Development’s National Center for Environmental Assessment, Superfund Health Risk Technical Support Center (513-569-7300). i Sulfolane TABLE OF CONTENTS COMMONLY USED ABBREVIATIONS ................................................................................... iii BACKGROUND .............................................................................................................................1 DISCLAIMERS ...............................................................................................................................1 QUESTIONS REGARDING PPRTVS ...........................................................................................1 -

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

Effect of Enzymes on Strawberry Volatiles During Storage, at Different Ripeness
Effect of Enzymes on Strawberry Volatiles During Storage, at Different Ripeness Level, in Different Cultivars and During Eating Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Gulsah Ozcan Graduate Program in Food Science and Technology The Ohio State University 2010 Thesis Committee: Sheryl Ann Barringer, Adviser W. James Harper John Litchfield 1 Copyright by Gülşah Özcan 2010 ii ABSTRACT Strawberry samples with enzyme activity and without enzyme activity (stannous chloride added) were measured for real time formation of lipoxygenase (LOX) derived aroma compounds after 5 min pureeing using selected ion flow tube mass spectrometry (SIFT-MS). The concentration of (Z)-3-hexenal and (E)-2-hexenal increased immediately after blending and gradually decreased over time while hexanal concentration increased for at least 5 min in ground strawberries. The formation of hexanal was slower than the formation of (Z)-3-hexenal and (E)-2-hexenal in the headspace of pureed strawberries. The concentration of LOX aldehydes and esters significantly increased during refrigerated storage. Damaging strawberries increased the concentration of LOX aldehydes but did not significantly affect the concentration of esters. The concentrations of many of the esters were strongly correlated to their corresponded acids and/or aldehydes. The concentration of LOX generated aldehydes decreased during ripening, while fruity esters increased. Different varieties had different aroma profiles and esters were the greatest percentage of the volatiles. The aroma release of some of the LOX derived aldehydes in the mouthspace in whole strawberries compared to chopped strawberries showed that these volatiles are formed in the mouth during chewing. -

Bioelectronic Nose Based on Single-Stranded DNA And
nanomaterials Article Bioelectronic Nose Based on Single-Stranded DNA and Single-Walled Carbon Nanotube to Identify a Major Plant Volatile Organic Compound (p-Ethylphenol) Released by Phytophthora Cactorum Infected Strawberries Hui Wang 1,2,* , Yue Wang 1, Xiaopeng Hou 2 and Benhai Xiong 1,* 1 State Key Laboratory of Animal Nutrition, Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing 100193, China; [email protected] 2 Research Institute of Wood Industry, Chinese Academy of Forestry, Beijing 100091, China; [email protected] * Correspondence: [email protected] or [email protected] (H.W.); [email protected] (B.X.); Tel.: +86-010-62811680 (B.X.) Received: 5 February 2020; Accepted: 3 March 2020; Published: 7 March 2020 Abstract: The metabolic activity in plants or fruits is associated with volatile organic compounds (VOCs), which can help identify the different diseases. P-ethylphenol has been demonstrated as one of the most important VOCs released by the Phytophthora cactorum (P. cactorum) infected strawberries. In this study, a bioelectronic nose based on a gas biosensor array and signal processing model was developed for the noninvasive diagnostics of the P.cactorum infected strawberries, which could overcome the limitations of the traditional spectral analysis methods. The gas biosensor array was fabricated using the single-wall carbon nanotubes (SWNTs) immobilized on the surface of field-effect transistor, and then non-covalently functionalized with different single-strand DNAs (ssDNA) through π–π interaction. The characteristics of ssDNA-SWNTs were investigated using scanning electron microscope, atomic force microscopy, Raman, UV spectroscopy, and electrical measurements, indicating that ssDNA-SWNTs revealed excellent stability and repeatability. -

Original Paper Comparison of Volatile Compounds in 'Fuji' Apples in The
_ Food Science and Technology Research, 23 (1), 79 89, 2017 Copyright © 2017, Japanese Society for Food Science and Technology doi: 10.3136/fstr.23.79 http://www.jsfst.or.jp Original paper Comparison of Volatile Compounds in ‘Fuji’ Apples in the Different Regions in China 1 2* 3 2 2 2 Ling QIN , Qin-Ping WEI , Wen-Huai KANG , Qiang ZHANG , Jian SUN and Song-Zhong LIU 1Department of Life Science, Hebei Normal University of Science and Technology, Qinhuangdao 066004 P. R. China 2Institute of Forestry & Pomology, Beijing Academy of Agriculture & Forestry Sciences,Beijing 100093 P.R. China 3College of Bioscience and Bioengineering, Hebei University of Science and Technology, Shijiazhuang 050018 P.R. China Received May 25, 2015 ; Accepted September 28, 2016 The characteristics of the volatiles from 43 ‘Fuji’ apples representing 14 different apple production regions in China were investigated using headspace-solid phase micro-extraction (HS-SPME) combined with gas chromatography–mass spectrometry (GC-MS). The results obtained from this experiment showed that sixty- four volatile compounds were identified in ‘Fuji’ apples collected from 43 counties in China. The major volatile compounds were identified as 2-methyl butyl acetate and hexyl acetate. The composition of volatiles and their contents in ‘Fuji’ apples varied in different regions. All of the ‘Fuji’ apple samples could be classified into the following groups using a principal component analysis of the volatiles: (1) apples with high concentrations of hexyl acetate and (Z)-3-hexenyl acetate, -

Groundwater and Soil Oxygen and Nutrients (Soil Tilling, Blowers) (Biogenie, 2006)
RemTech 2016 Brent Lennox, M.Sc., P.Geol., Senior Hydrogeologist Eric Pringle, M.A.Sc., P.Eng., Principal Hydrogeological Engineer Waterline Resources Inc. Used in Sulfinol for sour gas sweetening since 1960s Human health related guidelines Poorly adsorbed to soil High solubility in water Microbial degradation slow in typical groundwater conditions Clear, colourless, no field indicators (visual or olefactory) Microbial degradation rapid in aerobic environments and surface water (CCME, 2006) + 2- C4H8O2S + 6.5O2 4CO2 + 3H2O + 2H +SO4 Nutrients improve degradation times Low pH conditions inhibit degradation Typical degradation times: 2 to 4 days at 28°C and 8 to 12 days at 8°C (Green et al., 1998), average air temperatures during trials ranged from 6.9 to 14.1°C Groundwater and Soil Oxygen and nutrients (soil tilling, blowers) (Biogenie, 2006) Groundwater Activated Sludge Treatment System (WorleyParsons Komex, 2008) Oxidants (e.g., hydrogen peroxide) and/or UV light Mixed success (Barr Engineering, 2013; Gallegos et al., 2013; EBA, 2015) Peroxide and iron catalyst shown to be more effective than peroxide (Gallegos, 2013) No sulphate as by-product Site is an operating gas plant located in southern Alberta Constructed in 1960s Sulfolane investigation and monitoring since 1994 No active facilities Downgradient of active facilities Majority of plant waste stored here before the 1980s Potential materials disposed: alumina catalyst, filters (compressor, sulfinol, salt water, glycol, solvent receiver), zeolite, etc. Cells -

Effect of Rutin on Gentamicin-Induced Ototoxicity in Rats: a Biochemical and Histopathological Examination
ORIGINAL ARTICLE ENT UPDATES 11(1):8-13 DOI: 10.5152/entupdates.2021.887158 Effect of Rutin on Gentamicin-Induced Ototoxicity in Rats: A Biochemical and Histopathological Examination Abstract Objective: Gentamicin is a broad-spectrum aminoglycoside antibiotic administered parenterally for moderate to severe gram-negative infections. Ototoxicity is an im- portant side effect that limits gentamicin use. The aim of this study is to investigate the effect of rutin on gentamicin-induced ototoxicity in rats biochemically and histo- pathologically. Methods: Distilled water was administered by oral gavage to healthy controls (HG) and cobalt administered group (GC). 50 mg/kg rutin was administered by oral gavage to rutin + gentamicin (RGG) group. After one hour, 100 mg/kg gentamicin was injected intraperitoneally (i.p) to the RGG and GC animal groups. This procedure was repeated once a day for 14 days. Results: Malondialdehyde (MDA), nuclear factor-κB(NF-κB), tumor necrosis factor alpha (TNF-α) and interleukin-1 beta(IL-1β) levels in the cochlear nerve tissue of gen- tamicin-treated animals were significantly higher compared to healthy controls and rutin + gentamicin treated rats. On the other hand, the amount of total Glutathione (tGSH) was significantly lower compared to the control and rutin group. Histopatho- logical examination revealed degenerated myelinated nerve fibers in the gentamicin group and Schwann cell nuclei were generally not seen. There was a high accumula- tion of collagen fiber in the tissue and dilated blood capillaries. In the rutin group, my- elinated nerve fibers mostly exhibited normal morphology, Schwann cell nuclei were İsmail Salcan1 evident and the vessels were normal. -

Aldrich Raman
Aldrich Raman Library Listing – 14,033 spectra This library represents the most comprehensive collection of FT-Raman spectral references available. It contains many common chemicals found in the Aldrich Handbook of Fine Chemicals. To create the Aldrich Raman Condensed Phase Library, 14,033 compounds found in the Aldrich Collection of FT-IR Spectra Edition II Library were excited with an Nd:YVO4 laser (1064 nm) using laser powers between 400 - 600 mW, measured at the sample. A Thermo FT-Raman spectrometer (with a Ge detector) was used to collect the Raman spectra. The spectra were saved in Raman Shift format. Aldrich Raman Index Compound Name Index Compound Name 4803 ((1R)-(ENDO,ANTI))-(+)-3- 4246 (+)-3-ISOPROPYL-7A- BROMOCAMPHOR-8- SULFONIC METHYLTETRAHYDRO- ACID, AMMONIUM SALT PYRROLO(2,1-B)OXAZOL-5(6H)- 2207 ((1R)-ENDO)-(+)-3- ONE, BROMOCAMPHOR, 98% 12568 (+)-4-CHOLESTEN-3-ONE, 98% 4804 ((1S)-(ENDO,ANTI))-(-)-3- 3774 (+)-5,6-O-CYCLOHEXYLIDENE-L- BROMOCAMPHOR-8- SULFONIC ASCORBIC ACID, 98% ACID, AMMONIUM SALT 11632 (+)-5-BROMO-2'-DEOXYURIDINE, 2208 ((1S)-ENDO)-(-)-3- 97% BROMOCAMPHOR, 98% 11634 (+)-5-FLUORODEOXYURIDINE, 769 ((1S)-ENDO)-(-)-BORNEOL, 99% 98+% 13454 ((2S,3S)-(+)- 11633 (+)-5-IODO-2'-DEOXYURIDINE, 98% BIS(DIPHENYLPHOSPHINO)- 4228 (+)-6-AMINOPENICILLANIC ACID, BUTANE)(N3-ALLYL)PD(II) CL04, 96% 97 8167 (+)-6-METHOXY-ALPHA-METHYL- 10297 ((3- 2- NAPHTHALENEACETIC ACID, DIMETHYLAMINO)PROPYL)TRIPH 98% ENYL- PHOSPHONIUM BROMIDE, 12586 (+)-ANDROSTA-1,4-DIENE-3,17- 99% DIONE, 98% 13458 ((R)-(+)-2,2'- 963 (+)-ARABINOGALACTAN BIS(DIPHENYLPHOSPHINO)-1,1'- -

An Overview on “Stages of Anesthesia and Some Novel General Anesthetics Drug”
Rohit Jaysing Bhor. et al. /Asian Journal of Research in Chemistry and Pharmaceutical Sciences. 5(4), 2017, 132-140. Review Article ISSN: 2349 – 7106 Asian Journal of Research in Chemistry and Pharmaceutical Sciences Journal home page: www.ajrcps.com AN OVERVIEW ON “STAGES OF ANESTHESIA AND SOME NOVEL GENERAL ANESTHETICS DRUG” Rohit Jaysing Bhor *1 , Bhadange Shubhangi 1, C. J. Bhangale 1 1* Department of Pharmaceutical Chemistry, PRES’s College of Pharmacy Chincholi, Tal-Sinner, Dist-Nasik, Maharashtra, India. ABSTRACT Anesthesia is a painless performance of medical producers. There are both major and minor risks of anesthesia. Anesthesia is a state of temporary induced loss of sensation or awareness. It gives analgesia i.e. relief from pain or prevention of pain and paralysis. General anaesthesia is a medically induced state of unconsciousness. It gives loss of protective reflux. It is carried out to allow medical procedures or medical surgery. It can be classified into 3 types like Intravenous Anesthetics Drug; Miscellaneous Drug; and Inhalational anesthetic Drug. Sodium thiopental is an ultra-short-acting barbiturate and has been used commonly in the induction phase of anesthesia. Methohexital is an example of barbiturates derivatives. It is classified as short-acting, and has a rapid onset of action. KEYWORDS Anesthesia, Sodium thiopental, Methohexital and Propofol. Author for Correspondence: INTRODUCTON Anesthesia is a state of temporary induced loss of sensation or awareness. It gives analgesia i.e. relief 1 Rohit Jaysing Bhor, from pain or prevention of pain and paralysis . A patient under the effects of anesthetic drug is known Department of Pharmaceutical Chemistry, as anesthetized. -

Reactions of Some 1-Arylpiperazines and 1-Aryl-4-(2-Hydroxy-3
Reactions of Some l-Arylpiperazines and -Aryl-4- (2-Hydroxy-3-Methoxypropyl) Piperazines By HILDA HOWELL A DISSERTATION PRESENTED TO THE GRADUATE COUNQL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA August, 1961 5 ACKNOWLEDGMENTS The author would like to thank Parke, Davis and Company who provided a graduate fellowship during part of this work. The able assistance of the faculty, staff, and fellow graduate students of the Chemistry Department has made this research quite pleasant. Thanks are also due to Miss Joan Merritt, Mrs. Marie Eckart, Mr. T. W. Brooks, and Mr. Ralph Damico for their help with the typing, drawings, and proofreading of this report. The special consideration of Mr. P. L. Bhatia who shared this laboratory is appreciated. Special recognition is offered to D». C. B. Smith, Commdr. N. L. Smith, Dr. W. M. Jones, and Dr. G. B. Butler, each of whom from his own ability and opportunity has been of inestimable help. The moral support of her parents, family, and friends has significantly contributed to the success of this graduate program. She is deeply indebted to Dr* C. B. Pollard for his inspiration and direction !n the early part of this work and to her graduate ccKnmittee for their friendship demonstrated particularly since his death. She is especially indebted to Dr. Harry H. Slsler for the under- standing way in which he has directed the completion of this research. His friexidship and encouragement have made graduate work an enjoyable experience. ^ TABLE OF CONTENTS Page ACKNOWLEDGMENTS. -

(12) STANDARD PATENT (11) Application No. AU 2010224615 B2 (19) AUSTRALIAN PATENT OFFICE
(12) STANDARD PATENT (11) Application No. AU 2010224615 B2 (19) AUSTRALIAN PATENT OFFICE (54) Title Dietary supplement (51) International Patent Classification(s) A23K 1/175 (2006.01) A23K1/18 (2006.01) (21) Application No: 2010224615 (22) Date of Filing: 2010.02.12 (87) WIPONo: WO10/106351 (30) Priority Data (31) Number (32) Date (33) Country 0904584.0 2009.03.17 GB (43) Publication Date: 2010.09.23 (44) Accepted Journal Date: 2014.05.08 (71) Applicant(s) Calinnova Ltd (72) Inventor(s) Green, Malcolm Geoffrey (74) Agent / Attorney Davies Collison Cave, Level 14 255 Elizabeth Street, Sydney, NSW, 2000 (56) Related Art ARNBJERG, J. "Hypokalcaemi hos hest. En kasuistik med diskussion (Hypocalcemia in the horse. A case report)" Nord Vet Med, 1980, Volume 32, Issue 5 Pages 207-211 US 2003/0077254 A1 (RAMAEKERS) 24 April 2003 FR 2625415 A1 (UNICOR UNION COOP AGRICOLES) 07 July 1989 HUDSON, NPH et al., "Primary Hypothyroidism in Two Horses" Australian Veterinary Journal, 1999, Volume 77, Number 8, Pages 504-508 GRUBB, T.L. et al., "Hemodynamic Effects of Calcium Gluconate Administered to Conscious Horses" Journal of Veterinary Internal Medicine, 1996, Volume 10 Number 6 Pages 401-404 US 2008/0014304 A1 (ZELLER et al.) 17 January 2008 ANONYMOUS, "Finish Line Thia-cal" [Retrieved on 23 April 2013] Retrieved from internet <URL: http://www.doversaddlery.eom/finish-line-thia-cal-gallon/p/X1-22069/> published on 30 December 2005 ANONYMOUS, "Humavyte" [Retrieved on 23 April 2013] Retrieved from internet <URL: http://web.archive.org/web/20080719131829/http://www.ranvet.com.au/ -
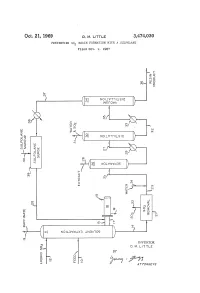
G ( NOW T LSO )
Oct. 21, 1969 D. M. E. 3,474,030 PREVENING SO RESIN FORMATION WITH A SULFOLANE Filed Oct. 9, 1967 f O O n S. NO WISO r W?? WA Q i? 1 a O r Q r C " ? l (? - 2 st e3 r g ( NOW T LSO ) SP & y (T)w 5 > < n Q - ( c O cy P O (? CO w o (f) 7: (s OW&WS ) O) O 3. R k N i 1 ? k y CO s m Yn C - CO s aa ( SS 2 Ele=E c l N 5: N NOLOW X3 NBA OS c INVENTOR. D. M. LTTLE BY y C Year A 77OAPAWAYS 3,474,030 United States Patent Office Patented Oct. 21, 1969 2 A further object of this invention is to minimize the 3,474,030 formation of resinous deposits during the operation of PREVENTING SO, RESIN FORMATION a unit employing SO2 and to provide a practical method WITH A SULFOLANE Donald M. Little, Bartlesville, Okla., assignor to Phillips for removing resinous materials inadvertently formed dur Petroleum Company, a corporation of Delaware 5 ing operation of the process. Filed Oct. 9, 1967, Ser. No. 673,607 Other objects, aspects as well as the several advantages Int. C. C10g 21/10, 21/22 of the invention will be apparent to those skilled in the U.S. C. 208-338 8 Claims art upon reading the specification, drawing, and the ap pended claims. O Summary of the invention ABSTRACT OF THE DISCLOSURE According to the invention, I have found that resinous Dissolution of resinous materials formed from SO2 and materials formed from SO2 and unsaturated materials unsaturated materials coming in contact with SO2, for ex contained in hydrocarbon oils coming in contact with SO ample, during the SO2 solvent extraction of hydrocarbon can be dissolved by contacting with at least one sulfolane.