Notes on the Growth and Ultrastructure of Biddulphia Laevis Ehr. (Bacillariophyceae) in the Maumee River, Ohio1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Page 1 03089500 Mill Creek Near Berlin Center, Ohio 19.13 40.9638 80.9476 10.86 9.13 0.6880 58.17 0.77 0.41 2.10 03092000 Kale C
Table 2-1. Basin characteristics determined for selected streamgages in Ohio and adjacent States. [Characteristics listed in this table are described in detail in the text portion of appendix 2; column headings used in this table are shown in parentheses adjacent to the bolded long variable names] Station number Station name DASS Latc Longc SL10-85 LFPath SVI Agric Imperv OpenWater W 03089500 Mill Creek near Berlin Center, Ohio 19.13 40.9638 80.9476 10.86 9.13 0.6880 58.17 0.77 0.41 2.10 03092000 Kale Creek near Pricetown, Ohio 21.68 41.0908 81.0409 14.09 12.88 0.8076 40.46 1.08 0.48 2.31 03092090 West Branch Mahoning River near Ravenna, Ohio 21.81 41.2084 81.1983 20.23 11.19 0.5068 38.65 2.35 1.01 2.51 03102950 Pymatuning Creek at Kinsman, Ohio 96.62 41.4985 80.6401 5.46 21.10 0.6267 52.26 0.82 1.18 5.60 03109500 Little Beaver Creek near East Liverpool, Ohio 495.57 40.8103 80.6732 7.89 55.27 0.4812 38.05 1.98 0.79 1.41 03110000 Yellow Creek near Hammondsville, Ohio 147.22 40.5091 80.8855 9.37 33.62 0.5439 19.84 0.34 0.33 0.36 03111500 Short Creek near Dillonvale, Ohio 122.95 40.2454 80.8859 15.25 27.26 0.3795 30.19 1.08 0.93 1.16 03111548 Wheeling Creek below Blaine, Ohio 97.60 40.1274 80.9477 13.43 27.46 0.3280 40.92 0.97 0.56 0.64 03114000 Captina Creek at Armstrongs Mills, Ohio 133.69 39.9307 81.0696 13.56 26.99 0.6797 32.76 0.54 0.64 0.66 03115400 Little Muskingum River at Bloomfield, Ohio 209.94 39.6699 81.1370 5.50 44.84 0.7516 10.00 0.25 0.12 0.12 03115500 Little Muskingum River at Fay, Ohio 258.25 39.6406 81.1531 4.32 60.10 0.7834 -

Ohio Archaeological Inventory Form Instruction Manual
Ohio Archaeological Inventory Form Instruction Manual With the support of the U.S. Department of the Interior’s Historic Preservation Fund and the Ohio Historic Preservation Office of the Ohio Historical Society Copyright © 2007 Ohio Historical Society, Inc. All rights reserved. The publication of these materials has been made possible in part by a grant from the U.S. Department of the Interior’s National Park Service, administered by the Ohio Historic Preservation Office. However, its contents do not necessarily reflect the opinions of the Department of the Interior, nor does the mention of trade names or commercial products imply their endorsement. The Ohio Historic Preservation Office receives federal assistance from the U.S. Department of the Interior’s Historic Preservation Fund. U.S. Department of the Interior regulations prohibit unlawful discrimination in depart- mental federally assisted programs on the basis of race, color, national origin, age or disability. Any person who believes he or she has been discriminated against in any program, activity, or facility operated by a recipient of Federal assistance should write to: Office of Equal Opportunity, U.S. Department of the Interior, National Park Service, 1849 C Street N.W., Washington D.C. 20240. Ohio Historic Preservation Office 567 East Hudson Street Columbus, Ohio 43211-1030 614/ 298-2000 Fax 614/ 298-2037 Visit us at www.ohiohistory.org OAl Rev. June 2003 Table of Contents Introduction and General Instructions 1 Definition of Archaeological Resource (Site) 1 Submitting an Ohio Archaeological Inventory Form 2 Itemized Instructions 3 A. Identification 3 1. Type of Form 3 2. -
SAP Crystal Reports
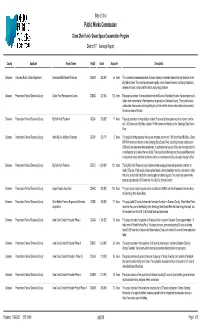
State of Ohio {rpt0010-17} Public Works Commission Clean Ohio Fund - Green Space Conservation Program District 17 Acreage Report County Applicant Project Name ProjID Grant Acquired Description Delaware Columbus Public Utilities Department Overbrook Multi-Stream ProtectionCQGAA 262,50055 Acres This conservation easement protects 55 acres of primary headwater streams that are tributaries to the Big Walnut Creek. The project protects water quality, natural stream channels, functioning floodplains, streamside forests, various wildlife habitat, and geologic features. Delaware Preservation Parks of Delaware County Gallant Farm Purchase and AccessCQBAD 327,503102 Acres The project purchases 19 acres and deed restricts 83 acres of farmland for use in the development of a historic farm representative of the importance of agriculture to Delaware County. The project includes construction of an access road and parking lot, and internal farm lanes to allow visitors easy access to the various areas of the farm. Delaware Preservation Parks of Delaware County Big Run North Purchase CQCAI 518,38711 Acres The project provides for the protection of about 11 acres of pristine upland woods and stream corridor and 1,482 linear feet of Big Run, a Class III PHWH stream and tributary to the Olentangy State Scenic River. Delaware Preservation Parks of Delaware County North Big Run Additional PurchaseCQDAF 659,1715 Acres This project is for the purchase of five acres of stream corridor and 1,834 linear feet of Big Run, a Class III PHWH stream and tributary to the Olentangy State Scenic River, a building that was constructed in 1939 which has been renovated andenlarged, a combination garage and office, and the construction of a small parking lot to service the new facility. -

Historic American Indian Tribes of Ohio 1654-1843
Historic American Indian Tribes of Ohio 1654-1843 Ohio Historical Society www.ohiohistory.org $4.00 TABLE OF CONTENTS Historical Background 03 Trails and Settlements 03 Shelters and Dwellings 04 Clothing and Dress 07 Arts and Crafts 08 Religions 09 Medicine 10 Agriculture, Hunting, and Fishing 11 The Fur Trade 12 Five Major Tribes of Ohio 13 Adapting Each Other’s Ways 16 Removal of the American Indian 18 Ohio Historical Society Indian Sites 20 Ohio Historical Marker Sites 20 Timeline 32 Glossary 36 The Ohio Historical Society 1982 Velma Avenue Columbus, OH 43211 2 Ohio Historical Society www.ohiohistory.org Historic American Indian Tribes of Ohio HISTORICAL BACKGROUND In Ohio, the last of the prehistoric Indians, the Erie and the Fort Ancient people, were destroyed or driven away by the Iroquois about 1655. Some ethnologists believe the Shawnee descended from the Fort Ancient people. The Shawnees were wanderers, who lived in many places in the south. They became associated closely with the Delaware in Ohio and Pennsylvania. Able fighters, the Shawnees stubbornly resisted white pressures until the Treaty of Greene Ville in 1795. At the time of the arrival of the European explorers on the shores of the North American continent, the American Indians were living in a network of highly developed cultures. Each group lived in similar housing, wore similar clothing, ate similar food, and enjoyed similar tribal life. In the geographical northeastern part of North America, the principal American Indian tribes were: Abittibi, Abenaki, Algonquin, Beothuk, Cayuga, Chippewa, Delaware, Eastern Cree, Erie, Forest Potawatomi, Huron, Iroquois, Illinois, Kickapoo, Mohicans, Maliseet, Massachusetts, Menominee, Miami, Micmac, Mississauga, Mohawk, Montagnais, Munsee, Muskekowug, Nanticoke, Narragansett, Naskapi, Neutral, Nipissing, Ojibwa, Oneida, Onondaga, Ottawa, Passamaquoddy, Penobscot, Peoria, Pequot, Piankashaw, Prairie Potawatomi, Sauk-Fox, Seneca, Susquehanna, Swamp-Cree, Tuscarora, Winnebago, and Wyandot. -

1 New Ohio and Indiana Records of Aquatic Insects (Ephemeroptera
Ohio Biological Survey Notes 9: 1–15, 2019. © Ohio Biological Survey, Inc. New Ohio and Indiana Records of Aquatic Insects (Ephemeroptera, Plecoptera, Trichoptera, Coleoptera: Elmidae, Diptera: Chironomidae) MICHAEL J. BOLTON1, SARAH K. MACY2, R. EDWARD DEWALT3, AND LUKE M. JACOBUS4 1Ohio Environmental Protection Agency, Division of Surface Water, 4675 Homer Ohio Lane, Groveport, OH 43125, Michael.Bolton@epa. ohio.gov; 2Formerly with the Ohio Environmental Protection Agency; current e-mail: [email protected]; 3University of Illinois, Illinois Natural History Survey, 1816 S Oak St., Champaign, IL 61820, [email protected]; 4Indiana University–Purdue University Columbus, 4601 Central Avenue, Columbus, IN 47203, [email protected]. Abstract: New state records and additional locations for rarely collected species are reported for Ephemeroptera (mayflies), Plecoptera (stoneflies), Trichoptera (caddisflies), Coleoptera: Elmidae (riffle beetles), and Diptera: Chironomidae (chironomids, non-biting midges, midges). These specimen records result primarily from Ohio Environmental Protection Agency biomonitoring of Ohio streams and from records found in the Purdue University Entomological Research Collection and the Illinois Natural History Survey Insect Collection; a few records were derived from material housed in two other collections. New state records for Ohio consist of the mayflies Acentrella rallatoma Burian & Myers, Acerpenna pygmaea (Hagen), Anafroptilum album (McDunnough), Anafroptilum minor group species 1, Anafroptilum -

Basin Descriptions and Flow Characteristics of Ohio Streams
Ohio Department of Natural Resources Division of Water BASIN DESCRIPTIONS AND FLOW CHARACTERISTICS OF OHIO STREAMS By Michael C. Schiefer, Ohio Department of Natural Resources, Division of Water Bulletin 47 Columbus, Ohio 2002 Robert Taft, Governor Samuel Speck, Director CONTENTS Abstract………………………………………………………………………………… 1 Introduction……………………………………………………………………………. 2 Purpose and Scope ……………………………………………………………. 2 Previous Studies……………………………………………………………….. 2 Acknowledgements …………………………………………………………… 3 Factors Determining Regimen of Flow………………………………………………... 4 Weather and Climate…………………………………………………………… 4 Basin Characteristics...………………………………………………………… 6 Physiology…….………………………………………………………… 6 Geology………………………………………………………………... 12 Soils and Natural Vegetation ..………………………………………… 15 Land Use...……………………………………………………………. 23 Water Development……………………………………………………. 26 Estimates and Comparisons of Flow Characteristics………………………………….. 28 Mean Annual Runoff…………………………………………………………... 28 Base Flow……………………………………………………………………… 29 Flow Duration…………………………………………………………………. 30 Frequency of Flow Events…………………………………………………….. 31 Descriptions of Basins and Characteristics of Flow…………………………………… 34 Lake Erie Basin………………………………………………………………………… 35 Maumee River Basin…………………………………………………………… 36 Portage River and Sandusky River Basins…………………………………….. 49 Lake Erie Tributaries between Sandusky River and Cuyahoga River…………. 58 Cuyahoga River Basin………………………………………………………….. 68 Lake Erie Tributaries East of the Cuyahoga River…………………………….. 77 Ohio River Basin………………………………………………………………………. 84 -

Low Impact Development Manual for the Lower Maumee and Ottawa River Watersheds
LOW IMPACT DEVELOPMENT MANUAL FOR THE LOWER MAUMEE AND OTTAWA RIVER WATERSHEDS © 2010 American Rivers PROJECT DIRECTORS Katie Swartz, American Rivers, Inc. Gary Belan, American Rivers, Inc. Funded by The Joyce Foundation Prepared by: Tetra Tech 420 Madison Avenue, Suite1001 Toledo, Ohio 43604-1258 Low Impact Development Center 4600 Powder Mill Road, Suite 200 Beltsville, Maryland 20705 Low Impact Development Manual i ACKNOWLEDGEMENTS We would like to acknowledge and thank the following people and organizations for their invaluable contributions to this manual: Patekka Banister, Beatrice Miringu, and Frank Mortali from the City of Toledo Jeff Grabarkiewicz from the Lucas County Soil and Water Conservation District Brian W. Miller from Lucas County Engineers Cheryl Rice from the United States Department of Agriculture – Natural Resources Conservation Service (USDA-NRCS) Patricia Tebbe of Ohio Environmental Protection Agency Jan Hunter of Naturally Native Nursery A special thank you goes out to The Joyce Foundation for their generous funding of this project. ii Low Impact Development Manual TABLE OF CONTENTS ACKNOWLEDGEMENTS ............................................................................................................ ii FOREWORD ................................................................................................................................. vi 1.0 INTRODUCTION ............................................................................................................ 1-1 1.1 Purpose of the Manual.................................................................................................. -

2019 America's Most Endangered Rivers ® Report
AMERICA’S MOST ENDANGERED RIVERS® 2019 2 019 America’s Most Climate change is the most Endangered consequential environmental Rivers® challenge facing our planet. Introduction Water scarcity due to global warming could displace 700 million people worldwide by 2030. In the same time frame, 54 million lives globally could be impacted by river floods. In the United States, our climate has warmed by 1.8 degrees in the past century. It’s time to get real about what this means: Forest fires are growing more destructive, cities are struggling to do more with less water, fish and wildlife are struggling to survive and storms are triggering more life-threatening floods. Moreover, the harshest impacts of climate change are often most prevalent in economically disadvantaged communities and communities of color. We must heed the warnings of the scientific community and quickly move away from reliance on dirty fossil fuels. And, we must unite for the clean water our families and ecosystems need to survive. Healthy rivers are key to protecting this life-giving resource for future generations. Rivers provide clean drinking water, water our crops, power our homes and businesses, provide wildlife habitat, reduce the severity of floods and droughts and provide wild places for us to fish, boat and explore. Today, our nation is at a crossroads: Will we act to protect rivers and strengthen our communities, or will we continue to exploit and damage our rivers, making ourselves more vulnerable to the impacts of climate change? America’s Most Endangered Rivers® of 2019 highlights what’s at stake and the critical choices we face for our future. -

Ohio EPA List of Special Waters April 2014
ist of Ohio’s Special Waters, As of 4/16/2014 Water Body Name - SegmenL ting Description Hydrologic Unit Special Flows Into Drainage Basin Code(s) (HUC) Category* Alum Creek - headwaters to West Branch (RM 42.8) 05060001 Big Walnut Creek Scioto SHQW 150 Anderson Fork - Grog Run (RM 11.02) to the mouth 05090202 Caesar Creek Little Miami SHQW 040 Archers Fork Little Muskingum River Central Ohio SHQW 05030201 100 Tributaries Arney Run - Black Run (RM 1.64) to the mouth 05030204 040 Clear Creek Hocking SHQW Ashtabula River - confluence of East and West Fork (RM 27.54) Lake Erie Ashtabula SHQW, State to East 24th street bridge (RM 2.32) 04110003 050 Scenic river Auglaize River - Kelly Road (RM 77.32) to Jennings Creek (RM Maumee River Maumee SHQW 47.02) 04100007 020 Auglaize River – Jennings Creek (RM 47.02) to Ottawa River (RM Maumee River Maumee Species 33.26) Aukerman Creek Twin Creek Great Miami Species Aurora Branch - State Route 82 (RM 17.08) to the mouth Chagrin River Chagrin OSW-E, State 04110003 020 Scenic river Bantas Fork Twin Creek Great Miami OSW-E 05080002 040 Baughman Creek 04110004 010 Grand River Grand SHQW Beech Fork 05060002 Salt Creek Scioto SHQW 070 Bend Fork – Packsaddle run (RM 9.7) to the mouth 05030106 110 Captina Creek Central Ohio SHQW Tributaries Big Darby Creek Scioto River Scioto OSW-E 05060001 190, 05060001 200, 05060001 210, 05060001 220 Big Darby Creek – Champaign-Union county line to U.S. route Scioto River Scioto State Scenic 40 bridge, northern boundary of Battelle-Darby Creek metro river park to mouth Big Darby Creek – Champaign-Union county line to Conrail Scioto River Scioto National Wild railroad trestle (0.9 miles upstream of U.S. -

An Evaluation of Habitat Structure and the Distribution of Rare and Common Darters in Ohio
Wright State University CORE Scholar Browse all Theses and Dissertations Theses and Dissertations 2011 An Evaluation of Habitat Structure and the Distribution of Rare and Common Darters in Ohio Erin Lee Kingdom Wright State University Follow this and additional works at: https://corescholar.libraries.wright.edu/etd_all Part of the Biology Commons Repository Citation Kingdom, Erin Lee, "An Evaluation of Habitat Structure and the Distribution of Rare and Common Darters in Ohio" (2011). Browse all Theses and Dissertations. 466. https://corescholar.libraries.wright.edu/etd_all/466 This Thesis is brought to you for free and open access by the Theses and Dissertations at CORE Scholar. It has been accepted for inclusion in Browse all Theses and Dissertations by an authorized administrator of CORE Scholar. For more information, please contact [email protected]. AN EVALUATION OF HABITAT STRUCTURE AND THE DISTRIBUTION OF RARE AND COMMON DARTERS IN OHIO A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By ERIN LEE KINGDOM B.S., Wright State University, 2004 2011 Wright State University WRIGHT STATE UNIVERSITY SCHOOL OF GRADUATE STUDIES June 20, 2011 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISION BY Erin L. Kingdom ENTITLED An Evaluation of Habitat Structure and the Distribution of Rare and Common Darters in Ohio BE ACCEPTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Science Yvonne Vadeboncoeur, Ph.D. Thesis Director David Goldstein, Ph. D. Department Chair of Biological Sciences Committee on Final Examination Yvonne Vadeboncoeur, Ph.D. James Runkle, Ph.D. -

Hydrologic Disturbance and Response of Aquatic Biota in Big Darby Creek Basin, Ohio
Hydrologic Disturbance and Response of Aquatic Biota in Big Darby Creek Basin, Ohio U.S. Geological Survey Water-Resources Investigations Report 96-4315 I Qidnrd Trch? Prepared in cooperation with The City of Columbus, Ohio, and Franklin, Madison, and Pickaway Counties COVER: Artistic representation of Big Darby Creek, Ohio WaterColor - Richard Frehs 1997 ERRATA in APPENDIX 1 Hambrook, J.A., Koltun, G.F., Palcsak, B.B., and Tertuliani, J.S. 1997. Hydrologic Disturbance and Response of Aquatic Biota in Big Darby Creek Basin, Ohio. U.S. Geological Survey, Water- Resources Investigations Report 96-4315, 79 p. p. 53 Branchiobdellida NOT Branchiobdellia; Elmidae NOT Elmimidae p. 55 Psephenidae NOT Asephenidae; Coenagrionidae NOT Coenagrioniidae p. 56 Neoperla clymene (Newman) NOT Neoperla cylmene (Newman) p. 60 Atrichopogon NOT Atrichipogon; Psychodidae NOT Psychadidae; Stratiomyidae NOT Stratiomyiidae p. 61 Antocha is a genus in Tipulidae NOT a Tabanidae; Rheocricotopus is an Orthocladiinae NOT Tanypodinae p. 63 Cricotopus vierriensis NOT C. vieriensis p. 64 Procladius is a Taaypodinae NOT Orthocladiinae; Tvetenia discoloripes NOT T. discoloipes Changes in family names since Merritt, R.W., and Cummins, K.W., eds., 1984, An introduction to the aquatic insects of North America (2d ed.): Dubuque, Iowa, Kendall Hunt, 722 p. p. 54 Lutrochus sp. belongs to Lutrochidae NOT Limnichidae p. 56 Isonychiidae NOT Oligoneuriidae. Prefered arrangement of the chironomids. Chironomidae Tanypodinae Podonominae Diamesinae Orthocladiinae Chironominae Chironomini Pseudochironomini Tanytarsini Hydrologic Disturbance and Response of Aquatic Biota in Big Darby Creek Basin, OHo By J.A. Hambrook, G.F. Koltun, B.B. Palcsak, and J.S. Tertuliani U.S. Geological Survey Water-Resources Investigations Report 96-4315 Prepared in cooperation with The City of Columbus, Ohio, and Franklin, Madison, and Pickaway Counties Columbus, Ohio 1997 U.S. -

Here Bank Stabilization Is Proposed
Peer Reviewed Publications Michael A. Hoggarth, Ph.D. 1 January 2019 Student co-authors in Bold Type Hoggarth, Michael A. and Michael Grumney. 2016. The Distribution and Abundance of Mussels (Bivalvia: Unionidae) in Lower Big Walnut Creek from Hoover Dam to its Mouth, in Franklin and Pickaway Counties, Ohio. Ohio Journal of Science, 116 (2): 48-59. Krebs, R. A., J. D. Hook, M. A. Hoggarth, and B. M. Walton. 2010. Evaluating the Mussel Fauna of the Chagrin River, A State-listed “Scenic” Tributary of Lake Erie. Northeastern Naturalist, 17(4): 565-574. Watters, G. Thomas, Michael A. Hoggarth and David. H. Stansbery. 2009. The Freshwater Mussels of Ohio. The Ohio State University Press. Columbus, Ohio. 421 pages. Hoggarth, Michael A., David A. Kimberly, and Benjamin G. Van Allen. 2007. A study of the mussels (Mollusca: Bivalvia: Unionidae) of Symmes Creek and tributaries in Jackson, Gallia and Lawrence counties, Ohio. The Ohio Journal of Science, 107 (4): 57-62. U.S. Fish and Wildlife Service. 2005. Revised Purple Catspaw Recovery Plan. U.S. Fish and Wildlife Service, Twin Cities, Minnesota. 38 p. (Prepared by Michael A. Hoggarth). U.S. Fish and Wildlife Service. 2005. Revised White Catspaw Recovery Plan. U.S. Fish and Wildlife Service, Twin Cities, Minnesota. 44 p. (Prepared by Michael A. Hoggarth). O’Brien, Christine A., James D. Williams, and Michael A. Hoggarth. 2003. Morphological variation in glochidia shells of six species of Elliptio from Gulf of Mexico and Atlantic Coast drainages in the southeastern Untied States. Proceedings of the Biological Society of Washington, 116(3):719-731.