Relationship of Soil Potassium Forms with Maize Potassium Contents in Soils Derived from Different Parent Materials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Punjab Roads Component
Due Diligence Report on Social Safeguards Loan 3264-PAK: Flood Emergency Reconstruction and Resilience Project (FERRP)–Punjab Roads Component Due Diligence Report on Social Safeguards on Reconstruction of Pasrur – Narowal Road March 2017 Prepared by: Communication and Works Department, Government of the Punjab NOTES (i) The fiscal year (FY) of the Government of the Islamic Republic of Pakistan and its agencies ends on 30 June. (ii) In this report, "$" refers to US dollars. This Social Safeguards due diligence report is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. Social Due Diligence Report Document stage: Final Date: March, 2017 PAK: Flood Emergency Reconstruction and Resilience Project, Loan No. 3264 Social Due Diligence Report of Reconstruction of 28 km long Pasrur – Narowal Road from RD 0+000 to RD 28+000), District Sialkot Prepared by: Abdul Hameed, TA Resettlement Specialist for Project Implementation Unit, Communications and Works Department, Government of Punjab, Lahore. This due diligence report is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of -

Village List of Gujranwala , Pakistan
Census 51·No. 30B (I) M.lnt.6-18 300 CENSUS OF PAKISTAN, 1951 VILLAGE LIST I PUNJAB Lahore Divisiona .,.(...t..G.ElCY- OF THE PROVINCIAL TEN DENT CENSUS, JUr.8 1952 ,NO BAHAY'(ALPUR Prleo Ps. 6·8-0 FOREWORD This Village List has been pr,epared from the material collected in con" nection with the Census of Pakistan, 1951. The object of the List is to present useful information about our villages. It was considered that in a predominantly rural country like Pakistan, reliable village statistics should be avaflable and it is hoped that the Village List will form the basis for the continued collection of such statistics. A summary table of the totals for each tehsil showing its area to the nearest square mile. and Its population and the number of houses to the nearest hundred is given on page I together with the page number on which each tehsil begins. The general village table, which has been compiled district-wise and arranged tehsil-wise, appears on page 3 et seq. Within each tehsil the Revenue Kanungo holqos are shown according to their order in the census records. The Village in which the Revenue Kanungo usually resides is printed in bold type at the beginning of each Kanungo holqa and the remaining Villages comprising the ha/qas, are shown thereunder in the order of their revenue hadbast numbers, which are given in column o. Rokhs (tree plantations) and other similar areas even where they are allotted separate revenue hadbast numbers have not been shown as they were not reported in the Charge and Household summaries. -

Appendix - II Pakistani Banks and Their Branches (December 31, 2008)
Appendix - II Pakistani Banks and their Branches (December 31, 2008) Allied Bank Ltd. Bhalwal (2) Chishtian (2) -Grain Market -Grain Market (743) -Noor Hayat Colony -Mohar Sharif Road Abbaspur 251 RB Bandla Bheli Bhattar (A.K.) Chitral Chungpur (A.K.) Abbottabad (4) Burewala (2) Dadu -Bara Towers, Jinnahabad -Grain Market -Pineview Road -Housing Scheme Dadyal (A.K) (2) -Supply Bazar -College Road -The Mall Chak Jhumra -Samahni Ratta Cross Chak Naurang Adda Johal Chak No. 111 P Daharki Adda Nandipur Rasoolpur Chak No. 122/JB Nurpur Danna (A.K.) Bhal Chak No. 142/P Bangla Danyor Adda Pansra Manthar Darband Adda Sarai Mochiwal Chak No. 220 RB Dargai Adda Thikriwala Chak No. 272 HR Fortabbas Darhal Gaggan Ahmed Pur East Chak No. 280/JB (Dawakhri) Daroo Jabagai Kombar Akalgarh (A.K) Chak No. 34/TDA Daska Arifwala Chak No. 354 Daurandi (A.K.) Attock (Campbellpur) Chak No. 44/N.B. Deenpur Bagh (A.K) Chak No. 509 GB Deh Uddhi Bahawalnagar Chak No. 76 RB Dinga Chak No. 80 SB Bahawalpur (5) Chak No. 88/10 R Dera Ghazi Khan (2) Chak No. 89/6-R -Com. Area Sattelite Town -Azmat Road -Dubai Chowk -Model Town -Farid Gate Chakwal (2) -Ghalla Mandi -Mohra Chinna Dera Ismail Khan (3) -Settelite Town -Talagang Road -Circular Road -Commissionery Bazar Bakhar Jamali Mori Talu Chaman -Faqirani Gate (Muryali) Balagarhi Chaprar Balakot Charsadda Dhamke (Faisalabad) Baldher Chaskswari (A.K) Dhamke (Sheikhupura) Bucheke Chattar (A.K) Dhangar Bala (A.K) Chhatro (A.K.) Dheed Wal Bannu (2) Dina -Chai Bazar (Ghalla Mandi) Chichawatni (2) Dipalpur -Preedy Gate -College Road Dir Barja Jandala (A.K) -Railway Road Dunyapur Batkhela Ellahabad Behari Agla Mohra (A.K.) Chilas Eminabad More Bewal Bhagowal Faisalabad (20) Bhakkar Chiniot (2) -Akbarabad Bhaleki (Phularwan Chowk) -Muslim Bazar (Main) -Sargodha Road -Chibban Road 415 ABL -Factory Area -Zia Plaza Gt Road Islamabad (23) -Ghulam Muhammad Abad Colony Gujrat (3) -I-9 Industrial Area -Gole Cloth Market -Grand Trunk Road -Aabpara -Gole Kiryana Bazar -Rehman Saheed Road -Blue Area ABL -Gulburg Colony -Shah Daula Road. -

Rawalpindi 1 Rawalpindi Leprosy Hospital, Zafar-Ul-Haq Road P.O Box No
Valid X-ray License Holder Sr. Facility Rawalpindi 1 Rawalpindi Leprosy Hospital, Zafar-ul-Haq Road P.O Box No. 135, Rawalpindi 2 Al-Shifa Eye Hospital, Jehlum Road, Rawalpindi 3 Health Ways Medical Centre, 8-111, Murree Road Opp. Ministry of Defence Secretrait, Saddar, Rawalpindi 4 Habib Hospital, Saidpur Road, Banni, Rawalpindi 5 Armed Forces Institute of Cadialogy(AFIC), The Mall Road, Rawalpindi 6 Christian Hospital, Faisal Shahid Road, Taxila, Rawalpindi 7 Islamic International Medical Complex Trust, Railway Hospital, Westridge, Rawalpindi 8 Abrar Surgery (Pvt.) Ltd, 219-B(1), Peshawar Road, Rawalpindi 9 Abrar Diagnostic Centre, 312-E, Chairing Cross Peshawar Road, Rawalpindi 10 WAPDA Hospital, Marrier Hassan, Rawalpindi 11 Combined Military Hospital, Murree, Rawalpindi 12 Attock Hospital(Pvt.) Ltd., P. O. Refinary Morgah, Rawalpindi 13 Kahuta Research Laboratories (KRL) Hospital, P. O. Sumbulgah, Tehsil Kahuta, Rawalpindi 14 KRL Medical Center, Dr. A. Q. Khan Road, P. O. Box 502, Rawalpindi 15 Urgent Medical Centre, Medical Tower, 101-A 6th Road, Satellite Town, Rawalpindi 16 Adil Diagnostics, B-1315, Saidpur Road, Rawalpindi 17 Valley Clinic (Pvt.) Ltd., 213, Peshawar Road, Lane 4, Rawalpindi 18 Maryam Memorial Hospital, Peshawar Road, Rawalpindi 19 Dr. Aslam Uppal Hospital & Labs, 3-Quaid Avenue Near Aslam Uppal Square, Lalazar, Wah Cantt., Rawalpindi 20 C/o Dr. Amenah Dental Clinic, 167/3A, Adamjee Road, Rawalpindi, Rawalpindi 21 Margalla Hospital, Pirwadahi Road, Pirwadahi, Rawalpindi 22 Al-Noor Dental Clinic, Mushtaq Plaza Holy Family Chowk Saidpur Raod, Rawalpindi 23 Hearts International Hospital, 192-A, The Mall, Rawalpindi 24 Al-Mustafa Trust Medical Center, Street No. 14, Mini Market Chaklala Scheme-III, Rawalpindi 25 Aman Hospital, 519/8-A, Misrial Road, Rawalpindi 26 DHQ Hospital, Raja Bazar, Rawalpindi 27 HIT Hospital, Taxila Cantt., Taxila, Rawalpindi 28 Syed Mohd Hussain Govt. -

Find Address of Your Nearest Loan Center and Phone Number of Concerned Focal Person
Find address of your nearest loan center and phone number of concerned focal person Loan Center/ S.No. Province District PO Name City / Tehsil Focal Person Contact No. Union Council/ Location Address Branch Name Akhuwat Islamic College Chowk Oppsite Boys College 1 Azad Jammu and Kashmir Bagh Bagh Bagh Nadeem Ahmed 0314-5273451 Microfinance (AIM) Sudan Galli Road Baagh Akhuwat Islamic Muzaffarabad Road Near main bazar 2 Azad Jammu and Kashmir Bagh Dhir Kot Dhir Kot Nadeem Ahmed 0314-5273451 Microfinance (AIM) dhir kot Akhuwat Islamic Mang bajri arja near chambar hotel 3 Azad Jammu and Kashmir Bagh Harighel Harighel Nadeem Ahmed 0314-5273451 Microfinance (AIM) Harighel Akhuwat Islamic 4 Azad Jammu and Kashmir Bhimber Bhimber Bhimber Arshad Mehmood 0346-4663605 Kotli Mor Near Muslim & School Microfinance (AIM) Akhuwat Islamic 5 Azad Jammu and Kashmir Bhimber Barnala Barnala Arshad Mehmood 0346-4663605 Main Road Bimber & Barnala Road Microfinance (AIM) Akhuwat Islamic Main choki Bazar near Sir Syed girls 6 Azad Jammu and Kashmir Bhimber Samahni Samahni Arshad Mehmood 0346-4663605 Microfinance (AIM) College choki Samahni Helping Hand for Adnan Anwar HHRD Distrcict Office Relief and Hattian,Near Smart Electronics,Choke 7 Azad Jammu and Kashmir Hattian Hattian UC Hattian Adnan Anwer 0341-9488995 Development Bazar, PO, Tehsil and District (HHRD) Hattianbala. Helping Hand for Adnan Anwar HHRD Distrcict Office Relief and Hattian,Near Smart Electronics,Choke 8 Azad Jammu and Kashmir Hattian Hattian UC Langla Adnan Anwer 0341-9488995 Development Bazar, PO, Tehsil and District (HHRD) Hattianbala. Helping Hand for Relief and Zahid Hussain HHRD Lamnian office 9 Azad Jammu and Kashmir Hattian Hattian UC Lamnian Zahid Hussain 0345-9071063 Development Main Lamnian Bazar Hattian Bala. -

1 Annexure - D Names of Village / Neighbourhood Councils Alongwith Seats Detail of Khyber Pakhtunkhwa
1 Annexure - D Names of Village / Neighbourhood Councils alongwith seats detail of Khyber Pakhtunkhwa No. of General Seats in No. of Seats in VC/NC (Categories) Names of S. Names of Tehsil Councils No falling in each Neighbourhood Village N/Hood Total Col Peasants/Work S. No. Village Councils (VC) S. No. Women Youth Minority . district Council Councils (NC) Councils Councils 7+8 ers 1 2 3 4 5 6 7 8 9 10 11 12 13 Abbottabad District Council 1 1 Dalola-I 1 Malik Pura Urban-I 7 7 14 4 2 2 2 2 Dalola-II 2 Malik Pura Urban-II 7 7 14 4 2 2 2 3 Dabban-I 3 Malik Pura Urban-III 5 8 13 4 2 2 2 4 Dabban-II 4 Central Urban-I 7 7 14 4 2 2 2 5 Boi-I 5 Central Urban-II 7 7 14 4 2 2 2 6 Boi-II 6 Central Urban-III 7 7 14 4 2 2 2 7 Sambli Dheri 7 Khola Kehal 7 7 14 4 2 2 2 8 Bandi Pahar 8 Upper Kehal 5 7 12 4 2 2 2 9 Upper Kukmang 9 Kehal 5 8 13 4 2 2 2 10 Central Kukmang 10 Nawa Sher Urban 5 10 15 4 2 2 2 11 Kukmang 11 Nawansher Dhodial 6 10 16 4 2 2 2 12 Pattan Khurd 5 5 2 1 1 1 13 Nambal-I 5 5 2 1 1 1 14 Nambal-II 6 6 2 1 1 1 Abbottabad 15 Majuhan-I 7 7 2 1 1 1 16 Majuhan-II 6 6 2 1 1 1 17 Pattan Kalan-I 5 5 2 1 1 1 18 Pattan Kalan-II 6 6 2 1 1 1 19 Pattan Kalan-III 6 6 2 1 1 1 20 Sialkot 6 6 2 1 1 1 21 Bandi Chamiali 6 6 2 1 1 1 22 Bakot-I 7 7 2 1 1 1 23 Bakot-II 6 6 2 1 1 1 24 Bakot-III 6 6 2 1 1 1 25 Moolia-I 6 6 2 1 1 1 26 Moolia-II 6 6 2 1 1 1 1 Abbottabad No. -

Audit Report on the Accounts of City District Government Rawalpindi Audit
AUDIT REPORT ON THE ACCOUNTS OF CITY DISTRICT GOVERNMENT RAWALPINDI AUDIT YEAR 2016-17 AUDITOR GENERAL OF PAKISTAN TABLE OF CONTENTS ABBREVIATIONS & ACRONYMS............................................................................................. I PREFACE...................................................................................................................................... III EXECUTIVE SUMMARY .......................................................................................................... IV SUMMARY TABLE & CHARTS ............................................................................................ VIII TABLE 1: AUDIT WORK STATISTICS ................................................................................... VIII TABLE 2: AUDIT OBSERVATIONS ........................................................................................ VIII TABLE3: OUTCOME STATISTICS ............................................................................................ IX TABLE4: IRREGULARITIES POINTED OUT ............................................................................. X TABLE 5 COST BENEFIT ............................................................................................................. X CHAPTER 1 .................................................................................................................................... 1 1.1 CITY DISTRICT GOVERNMENT RAWALPINDI ........................................................ 1 1.1.1 INTRODUCTION OF DEPARTMENTS ........................................................................ -

Indian Soldiers Died in Italy During World War II: 1943-45
Indian Soldiers died in Italy during World War II: 1943-45 ANCONA WAR CEMETERY, Italy Pioneer ABDUL AZIZ , Indian Pioneer Corps. Gurdaspur, Grave Ref. V. B. 1. Sepoy ABDUL JABAR , 10th Baluch Regiment. Hazara, Grave Ref. V. B. 4. Sepoy ABDUL RAHIM , 11th Indian Inf. Bde. Jullundur, Grave Ref. V. D. 6. Rifleman AITA BAHADUR LIMBU , 10th Gurkha Rifles,Dhankuta, Grave Ref. VII. D. 5. Sepoy ALI GAUHAR , 11th Sikh Regiment. Rawalpindi, Grave Ref. V. D. 4. Sepoy ALI MUHAMMAD , 11th Sikh Regiment, Jhelum, Grave Ref. V. B. 6. Cook ALLAH RAKHA , Indian General Service Corps,Rawalpindi, Grave Ref. III. L. 16. Sepoy ALTAF KHAN , Royal Indian Army Service Corps,Alwar, Grave Ref. V. D. 5. Rifleman ANAND KHATTRI, 2nd King Edward VII's Own Gurkha Rifles (The Sirmoor Rifles). Grave Ref. VII. B. 7. Sapper ARUMUGAM , 12 Field Coy., Queen Victoria's Own Madras Sappers and Miners. Nanjakalikurichi. Grave Ref. V. B. 2. Rifleman BAL BAHADUR ROKA, 6th Gurkha Rifles. , Grave Ref. VII. B. 5. Rifleman BAL BAHADUR THAPA, 8th Gurkha Rifles.,Tanhu, , Grave Ref. VII. D. 8. Rifleman BHAGTA SHER LIMBU , 7th Gurkha Rifles, Dhankuta, ,Grave Ref. VII. F. 1. Rifleman BHAWAN SING THAPA , 4th Prince of Wales' Own Gurkha Rifles. Gahrung, , Grave Ref. VII. C. 4. Rifleman BHIM BAHADUR CHHETRI , 6th Gurkha Rifles. Gorkha, Grave Ref. VII. C. 5. Rifleman BHUPAL THAPA , 2nd King Edward VII's Own Gurkha Rifles (The Sirmoor Rifles). Sallyan, Grave Ref. VII. E. 4. Rifleman BIR BAHADUR SUNWAR , 7th Gurkha Rifles. Ramechhap, Grave Ref. VII. F. 8. Rifleman BIR BAHADUR THAPA, 8th Gurkha Rifles, Palpa, Grave Ref. -
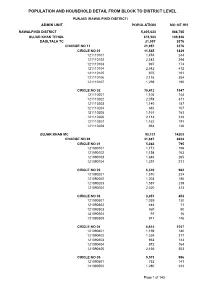
Rawalpindi Blockwise
POPULATION AND HOUSEHOLD DETAIL FROM BLOCK TO DISTRICT LEVEL PUNJAB (RAWALPINDI DISTRICT) ADMIN UNIT POPULATION NO OF HH RAWALPINDI DISTRICT 5,405,633 888,765 GUJAR KHAN TEHSIL 678,503 109,826 DAULTALA TC 21,957 3376 CHARGE NO 11 21,957 3376 CIRCLE NO 01 11,545 1829 121110101 1,474 244 121110102 2,143 294 121110103 997 174 121110104 2,542 412 121110105 975 161 121110106 2,116 354 121110107 1,298 190 CIRCLE NO 02 10,412 1547 121110201 1,105 144 121110202 2,078 311 121110203 1,140 187 121110204 682 107 121110205 1,167 163 121110206 2,114 318 121110207 1,162 191 121110208 964 126 GUJAR KHAN MC 90,131 14203 CHARGE NO 09 41,687 6634 CIRCLE NO 01 5,363 795 121090101 1,172 156 121090102 1,154 163 121090103 1,646 265 121090104 1,391 211 CIRCLE NO 02 6,320 962 121090201 1,510 224 121090202 1,203 186 121090203 1,587 239 121090204 2,020 313 CIRCLE NO 03 3,051 453 121090301 1,039 130 121090302 444 71 121090303 560 90 121090304 97 16 121090305 911 146 CIRCLE NO 04 6,614 1057 121090401 1,198 180 121090402 1,324 217 121090403 963 143 121090404 973 164 121090405 2,156 353 CIRCLE NO 05 5,573 996 121090501 752 141 121090502 1,280 233 Page 1 of 143 POPULATION AND HOUSEHOLD DETAIL FROM BLOCK TO DISTRICT LEVEL PUNJAB (RAWALPINDI DISTRICT) ADMIN UNIT POPULATION NO OF HH 121090503 1,083 196 121090504 1,313 233 121090505 1,145 193 CIRCLE NO 06 5,306 839 121090601 774 117 121090602 1,193 193 121090603 832 143 121090604 1,634 244 121090605 873 142 CIRCLE NO 07 5,736 921 121090701 1,128 178 121090702 547 104 121090703 1,670 261 121090704 2,391 378 CIRCLE NO 08 -

Appendices Appendix-I Number of Reporting Scheduled Banks and Their Branches (1980-2015)
Appendices Appendix-I Number of Reporting Scheduled Banks and Their Branches (1980-2015) As on Pakistani Banks Foreign Banks Total 30th June No. of Banks No. of Branches No. of Banks No. of Branches No. of Banks No. of Branches 1980 9 6,723 21 56 30 6,779 1981 9 7,103 21 56 30 7,159 1982 9 7,244 22 57 31 7,301 1983 9 7,195 23 59 32 7,254 1984 9 7,051 23 59 32 7,110 1985 9 6,980 23 59 32 7,039 1986 9 6,955 22 60 31 7,015 1987 9 7,023 23 63 32 7,086 1988 9 7,141 28 65 37 7,206 1989 10 7,188 25 66 34 7,254 1990 10 7,337 26 67 36 7,404 1991 10 7,480 26 69 36 7,549 1992 20 7,538 28 71 46 7,609 1993 20 7,634 27 71 47 7,705 1994 21 7,663 26 72 47 7,735 1995 25 8,200 25 73 50 8,273 1996 25 8,387 28 82 53 8,469 1997 25 8,446 27 84 52 8,530 1998 25 7,921 27 91 52 8,012 1999 25 7,841 27 94 52 7,935 2000 25 7,755 26 92 51 7,847 2001 24 7,165 25 88 49 7,253 2002 25 6,878 24 88 49 6,966 2003 24 6,834 22 82 46 6,916 2004 28 6,803 17 79 45 6,882 2005 28 7,014 17 91 45 7,105 2006 30 7,296 17 125 47 7,421 2007 34 7,693 13 64 47 7,757 2008 33 8,277 12 69 45 8,346 2009 33 8,686 13 97 46 8,783 2010 33 9,007 13 89 46 9,096 2011 32 9,341 12 58 44 9,399 2012 31 9,792 13 55 44 9,847 2013 31 10,332 7 29 38 10,361 2014 31 10,957 7 27 38 10,984 2015 30 11,705 5 11 35 11,716 120 Appendix-II Reporting Scheduled Banks &Their Branches by Group (June 30th ,2015) No. -
Rawalpindi Division NIC Applicantname Guardianname Address Winorder
Winner List Chief Minister Self Employment Scheme for Unemployed Educated Youth Rawalpindi Division NIC ApplicantName GuardianName Address WinOrder Distt. Attock Attock (Bolan) Key Used: sas91117 3710409582635 MUHAMMAD ASHFAQ MUHAMMAD NAWAZ P/O. GHARBI BASAL TEHSIL JAND 1 DISTT. ATTOCK 3710194732977 Muhammad Khalid Muhammad Idress House No.27, B Block Attock City. 2 3710117637067 IMRAN ALI HAJI SHER BAHADUR P/O. GOLRA DISTT. ATTOCK 3 3710167205235 IMTAZ AHMAD MUSTAQ AHMAD H NO. 381 SADDAR BAZZAR ATTOCK 4 CANTT 3710199615683 RAZI KHAN WARIS KHAN NEW ABADI QaSIM ABAD MIRZA P/O 5 SANJWAL CANTT DISTT 3710176505597 UMAIR AKHTAR AKHTAR HUSSAIN BHATTI H # 19 LANE # 2 6 GULHSANEKHUDADAD PHASE 5 3710126938959 ABDUL WAHEED AHMAD RUSTAM KHAN MOH.NASIR ABAD NEAR MASJID 7 KHATTAK FAROOQ E AZAM, DHOKE F 3710119531133 MUHAMMAD SALMAN IQBAL MUHAMMAD IQBAL MOHALA MADNI COLONY ZAFAR 8 ABBAD SANJWAL CANTT 3710130709617 JEHANGIR ABBAS GHULAM ABBAS H.NO.371, ST.NO.4, MOH. CHOIWEST 9 ATTOCK 3710117436007 MALIK RASHID MALIK FAZAL KHAN MOH. AMIN ABAD ST. NO.1 ATTOCK 10 3130235490949 Muhmmad Arshad Ghulam husain sial Mahalla Muhammad Abad p/o khan Bela 11 Teh. Liqauat p 3810408253813 M.SANA ULLAH JEEWAN KHAN GONDLAN WALA PO GOHAR WALA 12 TEH MANKERA 3710136961769 RIZWAN KHAN UMER KHAN D.S.G GATE MOHALLA SHAH NAGAR 13 SANWAL CANTT. DISTT. 3710117374349 MUHAMMAD DAUD MUHAMMAD FAROOQ MOHALA MUHAMMAD NAGAR SADAR 14 BAZAR ATTOCK 3710103070035 IMRAN ASLAM AWAN MUHAMAMD ASLAM AWAN MOH. NASIRABAD, H.NO.BXII 1525, 15 DHOKE FATEH ATTOCK 6110162800329 ARSHAD MEHMOOD MEHMOOD BLOCK NO.10 FLAT 14 CATEGORE V 16 SECTOR I-9/4 ISLAMA 3710107683223 FAISAL NAVEED NAVEED ISLAM VILLAGE MARI KANJOOR TEHSIL& 17 DISTT. -
Notice Inviting Tenders
NOTICE INVITING TENDERS. Sealed tenders are invited on percentage rates MRS (1stbi-Annual 1st Jan to 30 June 2021) item rates non-scheduled items, from the approved firms / contractors of Tehsil Council Gujar Khan / Civil Division, Rawalpindi (any Local Govt.) / Punjab Local Govt. Department, who have deposited their enlistment / renewal fee for the current Financial Year 2020-21. S.N0 Name of Scheme Estimated Earnest T.S No. Tender Cost in Money Fee Million 1. Construction of Path Nallah Jang UC Thathi 27/TO(I&S)/TC/GK 1.000 50000 2000 Dated 23.04.2021 2. Construction of Path Para Mohra & Street / 411/MO(I&S)/MCR/ Drain Gasroor Rajgan UC Thathi 1.750 87500 TC/GK Dated 2000 23.04.2021 3. Construction of Street House Nazir Kayani, 411/MO(I&S)/MCR/ Jhaangi& Street JanzaGahParan UC Thathi 1.650 82500 TC/GK Dated 2000 23.04.2021 4. Construction of Street MianaPotha UC 28/TO(I&S)/TC/GK 0.600 30000 2000 Thathi Dated 23.04.2021 5. Construction of Streets / Drain Baghdad 411/MO(I&S)/MCR/ Town, Rahoora, Dhoke Hashmat, 1.900 95000 TC/GK Dated 2000 MouzaMianiBorgi& UC Islam PuraJabbar 23.04.2021 6. Construction of Streets / Drain Palthiam, 29/TO(I&S)/TC/GK Mohra Mughlan (Ratala) & UC Islam 1.000 50000 2000 Dated 23.04.2021 PuraJabbar 7. Construction of Streets / Drain Mohra Vains, 30/TO(I&S)/TC/GK Dhoke Baqar Shah (Sohawa Mirza) & UC 0.800 40000 Dated 23.04.2021 2000 Islam PuraJabbar 8. Construction of Streets / Drain Dhoke 31/TO(I&S)/TC/GK Malkan (Harnoh), BalyamRajgan& UC Islam 0.800 40000 Dated 23.04.2021 2000 PuraJabbar 9.