Salicylates Amount Variation in Diferent Species of Lithuanian Willow
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synthesis of Aspirin
SYNTHESIS OF ASPIRIN I. OBJECTIVES AND BACKGROUND You will: synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify your product by recrystallization, perform a chemical test to identify the change in functional group from reactant to product, and determine the success of your synthesis by calculating the percentage yield of your product. INTRODUCTION Aspirin is one of the most widely used medications in the world. It is employed as an analgesic (pain relief), an anti-pyretic (fever control) and an anti-inflammatory. More recently, studies have indicated that daily intake of small doses of aspirin can lower the risk of heart attack and stroke in high-risk patients. The history of aspirin and its precursor dates back to ancient times. Documents attributed to Hippocrates, the father of modern medicine, from the 4th century B.C. refer to the alleviation of pain by chewing on the bark of a willow tree or ingesting a powder made from the bark and leaves of the willow. This remedy was passed on from generation to generation. Fast forward now to the 19th century, where the field of organic chemistry began to experience tremendous growth. By 1838, chemists had managed to isolate, purify and identify the component of willow bark that provided the analgesic benefit. The compound was named salicylic acid, which was based on the genus name of the willow. Efforts to market salicylic acid met with failure, due to an unfortunate side effect-- prolonged ingestion of salicylic acid led to stomach pain, and in some cases, ulcers. -

Pharmacokinetics of Salicylic Acid Following Intravenous and Oral Administration of Sodium Salicylate in Sheep
animals Article Pharmacokinetics of Salicylic Acid Following Intravenous and Oral Administration of Sodium Salicylate in Sheep Shashwati Mathurkar 1,*, Preet Singh 2 ID , Kavitha Kongara 2 and Paul Chambers 2 1 1B, He Awa Crescent, Waikanae 5036, New Zealand 2 School of Veterinary Sciences, College of Sciences, Massey University, Palmerston North 4474, New Zealand; [email protected] (P.S.); [email protected] (K.K.); [email protected] (P.C.) * Correspondence: [email protected]; Tel.: +64-221-678-035 Received: 13 June 2018; Accepted: 16 July 2018; Published: 18 July 2018 Simple Summary: Scarcity of non-steroidal anti-inflammatory drugs (NSAID) to minimise the pain in sheep instigated the current study. The aim of this study was to know the pharmacokinetic parameters of salicylic acid in New Zealand sheep after administration of multiple intravenous and oral doses of sodium salicylate (sodium salt of salicylic acid). Results of the study suggest that the half-life of the drug was shorter and clearance was faster after intravenous administration as compared to that of the oral administration. The minimum effective concentration required to produce analgesia in humans (16.8 µL) was achieved in sheep for about 0.17 h in the current study after intravenous administration of 100 and 200 mg/kg body weight of sodium salicylate. However, oral administration of these doses failed to achieve the minimum effective concentration as mentioned above. This study is of significance as it adds valuable information on pharmacokinetics and its variation due to breed, species, age, gender and environmental conditions. -

Table S1: Sensitivity, Specificity, PPV, NPV, and F1 Score of NLP Vs. ICD for Identification of Symptoms for (A) Biome Developm
Table S1: Sensitivity, specificity, PPV, NPV, and F1 score of NLP vs. ICD for identification of symptoms for (A) BioMe development cohort; (B) BioMe validation cohort; (C) MIMIC-III; (D) 1 year of notes from patients in BioMe calculated using manual chart review. A) Fatigue Nausea and/or vomiting Anxiety Depression NLP (95% ICD (95% CI) P NLP (95% CI) ICD (95% CI) P NLP (95% CI) ICD (95% CI) P NLP (95% CI) ICD (95% CI) P CI) 0.99 (0.93- 0.59 (0.43- <0.00 0.25 (0.12- <0.00 <0.00 0.54 (0.33- Sensitivity 0.99 (0.9 – 1) 0.98 (0.88 -1) 0.3 (0.15-0.5) 0.85 (0.65-96) 0.02 1) 0.73) 1 0.42) 1 1 0.73) 0.57 (0.29- 0.9 (0.68- Specificity 0.89 (0.4-1) 0.75 (0.19-1) 0.68 0.97 (0.77-1) 0.03 0.98 (0.83-1) 0.22 0.81 (0.53-0.9) 0.96 (0.79-1) 0.06 0.82) 0.99) 0.99 (0.92- 0.86 (0.71- 0.94 (0.79- 0.79 (0.59- PPV 0.96 (0.82-1) 0.3 0.95 (0.66-1) 0.02 0.95 (0.66-1) 0.16 0.93 (0.68-1) 0.12 1) 0.95) 0.99) 0.92) 0.13 (0.03- <0.00 0.49 (0.33- <0.00 0.66 (0.48- NPV 0.89 (0.4-1) 0.007 0.94 (0.63-1) 0.34 (0.2-0.51) 0.97 (0.81-1) 0.86 (0.6-0.95) 0.04 0.35) 1 0.65) 1 0.81) <0.00 <0.00 <0.00 F1 Score 0.99 0.83 0.88 0.57 0.95 0.63 0.82 0.79 0.002 1 1 1 Itching Cramp Pain NLP (95% ICD (95% CI) P NLP (95% CI) ICD (95% CI) P NLP (95% CI) ICD (95% CI) P CI) 0.98 (0.86- 0.24 (0.09- <0.00 0.09 (0.01- <0.00 0.52 (0.37- <0.00 Sensitivity 0.98 (0.85-1) 0.99 (0.93-1) 1) 0.45) 1 0.29) 1 0.66) 1 0.89 (0.72- 0.5 (0.37- Specificity 0.96 (0.8-1) 0.98 (0.86-1) 0.68 0.98 (0.88-1) 0.18 0.5 (0-1) 1 0.98) 0.66) 0.88 (0.69- PPV 0.96 (0.8-1) 0.8 (0.54-1) 0.32 0.8 (0.16-1) 0.22 0.99 (0.93-1) 0.98 (0.87-1) NA* 0.97) 0.98 (0.85- 0.57 (0.41- <0.00 0.58 (0.43- <0.00 NPV 0.98 (0.86-1) 0.5 (0-1) 0.02 (0-0.08) NA* 1) 0.72) 1 0.72) 1 <0.00 <0.00 <0.00 F1 Score 0.97 0.56 0.91 0.28 0.99 0.68 1 1 1 *Denotes 95% confidence intervals and P values that could not be calculated due to insufficient cells in 2x2 tables. -

The Role of Organic Small Molecules in Pain Management
molecules Review The Role of Organic Small Molecules in Pain Management Sebastián A. Cuesta and Lorena Meneses * Laboratorio de Química Computacional, Facultad de Ciencias Exactas y Naturales, Escuela de Ciencias Químicas, Pontificia Universidad Católica del Ecuador, Av. 12 de Octubre 1076 Apartado, Quito 17-01-2184, Ecuador; [email protected] * Correspondence: [email protected]; Tel.: +593-2-2991700 (ext. 1854) Abstract: In this review, a timeline starting at the willow bark and ending in the latest discoveries of analgesic and anti-inflammatory drugs will be discussed. Furthermore, the chemical features of the different small organic molecules that have been used in pain management will be studied. Then, the mechanism of different types of pain will be assessed, including neuropathic pain, inflammatory pain, and the relationship found between oxidative stress and pain. This will include obtaining insights into the cyclooxygenase action mechanism of nonsteroidal anti-inflammatory drugs (NSAID) such as ibuprofen and etoricoxib and the structural difference between the two cyclooxygenase isoforms leading to a selective inhibition, the action mechanism of pregabalin and its use in chronic neuropathic pain, new theories and studies on the analgesic action mechanism of paracetamol and how changes in its structure can lead to better characteristics of this drug, and cannabinoid action mechanism in managing pain through a cannabinoid receptor mechanism. Finally, an overview of the different approaches science is taking to develop more efficient molecules for pain treatment will be presented. Keywords: anti-inflammatory drugs; QSAR; pain management; cyclooxygenase; multitarget drug; Citation: Cuesta, S.A.; Meneses, L. cannabinoid; neuropathic pain The Role of Organic Small Molecules in Pain Management. -

Salsalate Tablets, USP 500 Mg and 750 Mg Rx Only
SALSALATE RX- salsalate tablet, film coated ANDAPharm LLC Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here. ---------- Salsalate Tablets, USP 500 mg and 750 mg Rx Only Cardiovascular Risk NSAIDs may cause an increase risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk. (See WARNINGS and CLINICAL TRIALS). Salsalate tablets, USP is contraindicated for the treatment of perioperative pain in the setting of coronary artery bypass graft (CABG) surgery (See WARNINGS). Gastrointestinal Risk NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events. (See WARNINGS). DESCRIPTION Salsalate, is a nonsteroidal anti-inflammatory agent for oral administration. Chemically, salsalate (salicylsalicylic acid or 2-hydroxybenzoic acid, 2-carboxyphenyl ester) is a dimer of salicylic acid; its structural formula is shown below. Chemical Structure: Inactive Ingredients: Colloidal Silicon Dioxide, D&C Yellow #10 Aluminum Lake, Hypromellose, Microcrystalline Cellulose, Sodium Starch Glycolate, Stearic Acid, Talc, Titanium Dioxide, Triacetin. CLINICAL PHARMACOLOGY Salsalate is insoluble in acid gastric fluids (<0.1 mg/mL at pH 1.0), but readily soluble in the small intestine where it is partially hydrolyzed to two molecules of salicylic acid. -
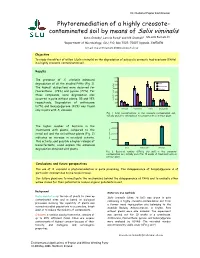
Phytoremediation of a Highly Creosote- Contaminated Soil by Means of Salix Viminalis
International Poplar Commission Phytoremediation of a highly creosote- contaminated soil by means of Salix viminalis Karin Önneby1, Leticia Pizzul1 and Ulf Granhall1 Mauritz Ramstedt 1Department of Microbiology, SLU, P.O. Box 7025, 75007 Uppsala, SWEDEN Email:E-mail: mauritz.ramstedt@m [email protected] Objective To study the effect of willow (Salix viminalis) on the degradation of polycyclic aromatic hydrocarbons (PAHs) in a highly creosote-contaminated soil. Results 1600 The presence of S. viminalis enhanced 1400 Initial degradation of all the studied PAHs (Fig. 1). 1200 The highest dissipations were observed for Without plant 1000 With plant fluoranthene (79%) and pyrene (77%). For 800 those compounds, some degradation also 600 occurred in pots without plants, 35 and 19% 400 Concentration (mg/kg dw) Concentration respectively. Degradation of anthracene 200 (67%) and benzo[a]pyrene (43%) was found 0 Anthracene Fluoranthene Pyrene Benzo[a]pyrene only in pots with S. viminalis. Fig. 1. PAH concentrations in the creosote-contaminated soil, initially and after 10 weeks of treatment with or without plant. 4000000 The higher number of bacteria in the 3000000 treatments with plants, compared to the initial soil and the soil without plants (Fig. 2) 2000000 indicates an increase in microbial activity. CFU (dw) /g soil 1000000 This activity, and possibly a higher release of biosurfactants, could explain the enhanced 0 degradation obtained with plants. Initial Without plant With plant Fig. 2. Bacterial number (CFU/g dry soil) in the creosote- contaminated soil, initially and after 10 weeks of treatment with or without plant. Conclusions and future perspectives The use of S. -

Poplars and Willows: Trees for Society and the Environment / Edited by J.G
Poplars and Willows Trees for Society and the Environment This volume is respectfully dedicated to the memory of Victor Steenackers. Vic, as he was known to his friends, was born in Weelde, Belgium, in 1928. His life was devoted to his family – his wife, Joanna, his 9 children and his 23 grandchildren. His career was devoted to the study and improve- ment of poplars, particularly through poplar breeding. As Director of the Poplar Research Institute at Geraardsbergen, Belgium, he pursued a lifelong scientific interest in poplars and encouraged others to share his passion. As a member of the Executive Committee of the International Poplar Commission for many years, and as its Chair from 1988 to 2000, he was a much-loved mentor and powerful advocate, spreading scientific knowledge of poplars and willows worldwide throughout the many member countries of the IPC. This book is in many ways part of the legacy of Vic Steenackers, many of its contributing authors having learned from his guidance and dedication. Vic Steenackers passed away at Aalst, Belgium, in August 2010, but his work is carried on by others, including mem- bers of his family. Poplars and Willows Trees for Society and the Environment Edited by J.G. Isebrands Environmental Forestry Consultants LLC, New London, Wisconsin, USA and J. Richardson Poplar Council of Canada, Ottawa, Ontario, Canada Published by The Food and Agriculture Organization of the United Nations and CABI CABI is a trading name of CAB International CABI CABI Nosworthy Way 38 Chauncey Street Wallingford Suite 1002 Oxfordshire OX10 8DE Boston, MA 02111 UK USA Tel: +44 (0)1491 832111 Tel: +1 800 552 3083 (toll free) Fax: +44 (0)1491 833508 Tel: +1 (0)617 395 4051 E-mail: [email protected] E-mail: [email protected] Website: www.cabi.org © FAO, 2014 FAO encourages the use, reproduction and dissemination of material in this information product. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

Nova Scotia Provincial Status Report on Hoary Willow Salix Candida
i Nova Scotia Provincial Status Report on Hoary Willow Salix candida Flűeggé ex Willd. prepared for The Nova Scotia Species at Risk Working Group by Ruth E. Newell E.C. Smith Herbarium K.C. Irving Environmental Science Centre Acadia University Wolfville, Nova Scotia B4P 2R6 Funding provided by the Nova Scotia Species at Risk Conservation Fund Submitted December 16th, 2010 ii EXECUTIVE SUMMARY Wildlife Species Description and Significance Salix candida (Hoary Willow) is a low, deciduous, dioecious shrub, densely white woolly on current season’s twigs and lower leaf surfaces. The mature medial leaves are narrowly elliptic or oblanceolate, usually at least 4x as long as wide. Leaf margins are entire and slightly to strongly rolled under. Flowering occurs concurrently with leaf emergence. Female flowers have stalks 0.1 to 1.2 mm long and tomentose pistils. The anthers of male flowers are purple later changing to yellow. The fruit is a tomentose, pear-shaped capsule. Reproduction is both sexual and asexual by layering. Salix candida is an extremely rare species in Nova Scotia occurring in a rare habitat type i.e., rich, calcareous fens or marshes. Distribution In Nova Scotia, Hoary Willow occurs within the Black River system at the northwest end of Lake Ainslie, Inverness County, Cape Breton Island. Here it is known from four rich calcareous fens in close proximity to the river floodplain plus a single plant in a calcareous graminoid marsh. Field work failed to confirm the presence of Salix candida in Huntington, Cape Breton County - a record based on a herbarium specimen from Cape Breton University herbarium. -

Cultivated Willows Would Not Be Appropriate Without Mention of the ‘WEEPING WILLOW’
Alexander Robertson Notes on willows cultivated in Scotland and a few sketches of willows sampled from my clonal collection HISTORICAL NOTES Since the knowledge of willows is of great antiquity, it is with the ancient Greeks and Romans we shall begin, for among these people numerous written records remain. The growth habit, ecology, cultivation and utilization of willows was well— understood by Theophrastus, Ovid, Herodotus, Pliny and Dioscorides. Virgil was also quite familiar with willow, e.g. Damoetas complains that: “Galatea, saucy girl, pelts me with apples and then runs off to the willows”. ECLOGIJE III and of foraging bees: “Far and wide they feed on arbutus, pale-green willows, on cassia and ruddy crocus .. .“ GEORGICS IV Theophrastus of Eresos (370—285 B.C.) discussed many aspects of willows throughout his Enquiry into Plants including habitats, wood quality, coppicing and a variety of uses. Willows, according to Theophrastus are lovers of wet places and marshes. But he also notes certain amphibious traits of willows growing in mountains and plains. To Theophrastus they appeared to possess no fruits and quite adequately reproduced themselves from roots, were tolerant to flooding and frequent coppicing. “Even willows grow old and when they are cut, no matter at what height, they shoot up again.” He described the wood as cold, tough, light and resilient—qualities which made it useful for a variety of purposes, especially shields. Such were the diverse virtues of willow that he suggested introducing it for plant husbandry. Theophrastus noted there were many different kinds of willows; three of the best known being black willow (Salix fragilis), white willow (S. -

Salix L.) in the European Alps
diversity Review The Evolutionary History, Diversity, and Ecology of Willows (Salix L.) in the European Alps Natascha D. Wagner 1 , Li He 2 and Elvira Hörandl 1,* 1 Department of Systematics, Biodiversity and Evolution of Plants (with Herbarium), University of Goettingen, Untere Karspüle 2, 37073 Göttingen, Germany; [email protected] 2 College of Forestry, Fujian Agriculture and Forestry University, Fuzhou 350002, China; [email protected] * Correspondence: [email protected] Abstract: The genus Salix (willows), with 33 species, represents the most diverse genus of woody plants in the European Alps. Many species dominate subalpine and alpine types of vegetation. Despite a long history of research on willows, the evolutionary and ecological factors for this species richness are poorly known. Here we will review recent progress in research on phylogenetic relation- ships, evolution, ecology, and speciation in alpine willows. Phylogenomic reconstructions suggest multiple colonization of the Alps, probably from the late Miocene onward, and reject hypotheses of a single radiation. Relatives occur in the Arctic and in temperate Eurasia. Most species are widespread in the European mountain systems or in the European lowlands. Within the Alps, species differ eco- logically according to different elevational zones and habitat preferences. Homoploid hybridization is a frequent process in willows and happens mostly after climatic fluctuations and secondary contact. Breakdown of the ecological crossing barriers of species is followed by introgressive hybridization. Polyploidy is an important speciation mechanism, as 40% of species are polyploid, including the four endemic species of the Alps. Phylogenomic data suggest an allopolyploid origin for all taxa analyzed Citation: Wagner, N.D.; He, L.; so far. -

Salix × Meyeriana (= Salix Pentandra × S
Phytotaxa 22: 57–60 (2011) ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ Correspondence PHYTOTAXA Copyright © 2011 Magnolia Press ISSN 1179-3163 (online edition) Salix × meyeriana (= Salix pentandra × S. euxina)―a forgotten willow in Eastern North America ALEXEY G. ZINOVJEV 9 Madison Ave., Randolph, MA 02368, USA; E-mail: [email protected] Salix pentandra L. is a boreal species native to Europe and western Siberia. In North America it is considered to have been introduced to about half of the US states (Argus 2007, 2010). In Massachusetts it is reported from nine of the fourteen counties (Sorrie & Somers 1999). Even though this plant may have been introduced to the US and Canada, its naturalization in North America appears to be quite improbable. Unlike willows from the related section Salix, in S. pentandra twigs are not easily broken off and their ability to root is very low, 0–15% (Belyaeva et al. 2006). It is possible to propagate S. pentandra from softwood cuttings (Belyaeva et al. 2006) and it can be cultivated in botanical gardens, however, vegetative reproduction of this species by natural means seems less likely. In North America this willow is known only by female (pistillate) plants (Argus 2010), so for this species, although setting fruit, sexual reproduction should be excluded. It is difficult to imagine that, under these circumstances, it could escape from cultivation. Therefore, most of the records for this willow in North America should be considered as collections from cultivated plants or misidentifications (Zinovjev 2008–2010). Salix pentandra is known to hybridize with willows of the related section Salix.