Exotic Pest Identification and Surveillance Guide for Tropical Horticulture (Version 1.0 February, 2021)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Records of Some Pests of the Coconut Inflorescence and Developing Fruit and Their Natural Enemies in Sri Lanka
COCOS, (1987) 5, 39—42 Printed in Sri Lanka New Records of some Pests of the Coconut Inflorescence and Developing Fruit and Their Natural Enemies in Sri Lanka L. C. P. FERNANDO and P. KANAGARATNAM Coconut Research Institute, Lunuwila, Sri Lanka. ABSTRACT A survey was carried out at Bandirippuwa Estate, Kirimetiyana Estate and at Isolated Seed Garden, Rajakadaluwa for the pests of coconut inflorescence and developing fruits. The mite, Dolichotetranychus sp. (Tenuipalpidae) which lives beneath the perianth and feeds on the epicarp, causes considerable damage to the developing fruit. Bi-monthly observations on the intensity of infestation of nuts among different forms of coconut indicated a significant difference among forms in their susceptibility to mite damage. Pseudococcus cocotis (Maskell) (Pseudococcidae), Pseudococcus citriculus Green (Pseudococcidae) and Planococcus lilacinus (Cockereli) (Pseudococcidae), when feeding in large numbers on the peduncle, cause button nut shedding and drying up of the inflo rescence. The parasitoids and predators of these mealybugs recorded for the first time in Sri Lanka are Promuscidea unfasciativentris Girault (Aphelinidae) Platygaster sp. (Platygastridae), Anagyrus sp. nr. pseudococci (Girault) (Encyrtidae) Coccodiplosis sp. (Cecidomyiidae), Cryptogonus bryanti Kapur (Coccinellidae) and Pseudoscymnus sp. (Coccinellidae). Several species of scale insects namely, Coccus hesperidum (Linnaeus) (Coccidae) Aulacaspis sp. (Diaspididae), Pseudaulacaspis cockerelli (Cooley) (Diaspididae), Aoni- diella orientalis (Newstead) (Diaspididae), and Pinnaspis strachani (Cooley) (Diaspididae) are recorded as minor pests of the developing fruits of coconut. Coccophagus silvestrii Compere (Aphelinidae), Scymnus sp. (Coccinellidae), Pseudoscymnus sp. (Coccinellidae) Telsimia ceylonica (Weise) (Coccinellidae), Cryptogonus bryanti Kapur (Coccinellidae) and Cybocephalus sp. (Nitidulidae) are recorded as their parasitoids and predators. These natural enemies are probably responsible for the control of these pests in the field. -

Insert Report Title Here
Vegetable biosecurity & quarantine gap analysis Prue McMichael Scholefield Robinson Horticultural Services Pty Ltd Project Number: VG07087 VG07087 This report is published by Horticulture Australia Ltd to pass on information concerning horticultural research and development undertaken for the vegetable industry. The research contained in this report was funded by Horticulture Australia Ltd with the financial support of the vegetable industry. All expressions of opinion are not to be regarded as expressing the opinion of Horticulture Australia Ltd or any authority of the Australian Government. The Company and the Australian Government accept no responsibility for any of the opinions or the accuracy of the information contained in this report and readers should rely upon their own enquiries in making decisions concerning their own interests. ISBN 0 7341 1849 X Published and distributed by: Horticulture Australia Ltd Level 7 179 Elizabeth Street Sydney NSW 2000 Telephone: (02) 8295 2300 Fax: (02) 8295 2399 E-Mail: [email protected] © Copyright 2008 FINAL REPORT Vegetable Biosecurity and Quarantine Gap Analysis VG07087 Prepared for : Horticulture Australia Ltd HAL Project No. VG07087 Prepared by : Prue McMichael Completion Date : September 2008 SCHOLEFIELD ROBINSON HORTICULTURAL SERVICES PTY LTD 118A Glen Osmond Road, Parkside SA 5063 Australia ACN 008 199 737 PO Box 650, Fullarton SA 5063 Ph: (08) 8373 2488 ABN 63 008 199 737 Fax: (08) 8373 2442 Email: [email protected] Web Site: www.srhs.com.au Offices in Adelaide and Mildura Scholefield Robinson Horticultural Services Pty Ltd HAL Project No. VG 07087 PROJECT LEADER Dr Prue McMichael Senior Consultant/Plant Pathologist Scholefield Robinson Horticultural Services Pty Ltd PO Box 650 Fullarton SA 5063 PURPOSE OF REPORT This Final Report has been prepared to document information acquired, analysed and considered during the review undertaken for HAL, into all aspects of the biosecurity of Australia’s vegetable industries that are members of AUSVEG. -

Molecular Basis of Pheromonogenesis Regulation in Moths
Chapter 8 Molecular Basis of Pheromonogenesis Regulation in Moths J. Joe Hull and Adrien Fónagy Abstract Sexual communication among the vast majority of moths typically involves the synthesis and release of species-specifc, multicomponent blends of sex pheromones (types of insect semiochemicals) by females. These compounds are then interpreted by conspecifc males as olfactory cues regarding female reproduc- tive readiness and assist in pinpointing the spatial location of emitting females. Studies by multiple groups using different model systems have shown that most sex pheromones are synthesized de novo from acetyl-CoA by functionally specialized cells that comprise the pheromone gland. Although signifcant progress was made in identifying pheromone components and elucidating their biosynthetic pathways, it wasn’t until the advent of modern molecular approaches and the increased avail- ability of genetic resources that a more complete understanding of the molecular basis underlying pheromonogenesis was developed. Pheromonogenesis is regulated by a neuropeptide termed Pheromone Biosynthesis Activating Neuropeptide (PBAN) that acts on a G protein-coupled receptor expressed at the surface of phero- mone gland cells. Activation of the PBAN receptor (PBANR) triggers a signal trans- duction cascade that utilizes an infux of extracellular Ca2+ to drive the concerted action of multiple enzymatic steps (i.e. chain-shortening, desaturation, and fatty acyl reduction) that generate the multicomponent pheromone blends specifc to each species. In this chapter, we provide a brief overview of moth sex pheromones before expanding on the molecular mechanisms regulating pheromonogenesis, and con- clude by highlighting recent developments in the literature that disrupt/exploit this critical pathway. J. J. Hull (*) USDA-ARS, US Arid Land Agricultural Research Center, Maricopa, AZ, USA e-mail: [email protected] A. -

31 First Record of Batocera Rufomaculata (De Geer, 1775) from Sunderban Biosphere Reserve, West Bengal
International Journal of Entomology Research ISSN: 2455-4758 www.entomologyjournals.com Volume 1; Issue 3; March 2016; Page No. 31-32 First record of Batocera rufomaculata (De Geer, 1775) from Sunderban biosphere reserve, West Bengal 1 Bulganin Mitra, 2 Udipta Chakraborti, 3 Olive Biswas, 4 Sankarsan Roy, 5 Kaushik Mallick, 6 Priyanka Das 1, 2, 3, 4, 6 Zoological Survey of India, Prani Vigyan Bhawan, M-Block, New Alipore, Kolkata. 5 Post Graduate Department of Zoology, Asutosh College, Kolkata Abstract Studies on Longhorn beetles (Coleoptera) in Sunderban region is very poor. Altogether, 8 species under 3 subfamilies are already reported from Sunderban Biosphere Reserve. Present communication reports Batocera rufomaculata (De Geer, 1775) for the first time from this Biosphere reserve. Keywords: Sunderban Biosphere Reserve, Cerambycidae, Lamiinae, Batocera Introduction Sunderban region in India is 9600 sq km (4200 sq km of Reserved Forest and 5400 sq km of non-forest, inhabited region) which constitutes the Sunderban Biosphere Reserve (SBR). Indian Sunderban is bound on the west by river Muriganga and on the east by rivers Harinbhahga and Raimangal. Administrative boundary of the Sunderban is spread over two districts i.e. North 24-Parganas (Hingalganj, Hasnabad, Haroa, Sandeskhali - I,II, and Minakhan blocks) and South 24-Parganas (Sagar, Namkhana, Kakdwip, Patharpratima, Kultali, Mathurapur-I,II, Jaynagar-I,II, Canning-I,II, Basanti and Gosaba blocks).The extent of mangrove Reserve Forests in Indian Sunderban is around 4260 sq km, out of which 55% is under land vegetation cover and balance 45% is under water body/ inter-tidal zone. Studies on beetles and weevils (Coleoptera) in Sunderban region is very poor. -

Assessment of Damage Caused by the Coconut Bug Pseudotheraptus Wayi (Brown) (Hemiptera: Coreidae) on Guavas
Fruits - vol . 47, n°2, 1992 31 7 Assessment of damage caused by the coconut bug Pseudotheraptus wayi (Brown) (Hemiptera: Coreidae) on guavas. T. VAN DER MEULEN * ASSESSMENT OF DAMAGE CAUSED BY THE COCONUT BUG EVALUATION DES DEGATS CAUSES PAR LE PARASITE DE S PSEUDOTHERAPTUS WAY! (BROWN) (HEMIPTERA : COCOTIERS , PSEUDOTHERAPTUS WAY! (BROWN) COREIDAE) ON GUAVAS . (HEMIPTERA : COREIDAE) SUR GOYAVES. T . VAN DER MEULEN . T. VAN DER MEULEN . Fruits, Mar .-Apr . 1992, vol . 47, n° 2, p .317-320 . Fruits, Mar : Apr . 1992, vol . 47, n° 2, p .317-320 . ABSTRACT - Damage caused by the coconut bug, Pseudotheraptu s RESUME - Sur goyaves, les dégâts causés par Pseudotheraptus wayi , wayi on guavas was studied in the Nelspruit Area . Three orchards, an d parasite du cocotier, ont été étudiés dans la région de Nelspruit . Dans 10 trees per orchard, were monitored weekly, from fruit drop unti l trois vergers, dix arbres par verger ont été observés chaque semaine , harvest . None of these orchards were sprayed with insecticides . de la nouaison à la récolte . Aucun de ces vergers na subi de traite- Average of 30 per cent of the aborted fruits and 26 per cent of th e ments insecticides . En moyenne, 30 p . 100 des fruits avortés et 26 p . ripe fruits were damaged . 100 des fruits parvenus à maturité ont été endommagés . INTRODUCTIO N readily when disturbed . Each individual makes about 200 punctures in the course of its existence . The insect lives i n The coconut bug, Pseudotheraptus wayi (Brown) (He- the crown of the palm throughout its life and the nymphs miptera : Coreidae) indigenous to East-Africa on coconut s are found only on or near the spadix . -

Stenoma Catenifer Walsingham
Avocado Seed Moth Screening Aid Stenoma catenifer Walsingham Hanna R. Royals1, Todd M. Gilligan1 and Steven C. Passoa2 1) Identification Technology Program (ITP) / Colorado State University, USDA-APHIS-PPQ-Science & Technology (S&T), 2301 Research Boulevard, Suite 108, Fort Collins, Colorado 80526 U.S.A. (Emails: [email protected]; [email protected]) 2) USDA-APHIS-PPQ, USDA-FS Northern Forest Research Station and Ohio State University, 1315 Kinnear Road, Columbus, Ohio 43212 U.S.A. (Email: [email protected]) This CAPS (Cooperative Agricultural Pest Survey) screening aid produced for and distributed by: Version 2 20 January 2016 USDA-APHIS-PPQ National Identification Services (NIS) This and other identification resources are available at: http://caps.ceris.purdue.edu/taxonomic_services The avocado seed moth, Stenoma catenifer is one of the most important moth pests in avocado-growing regions of the world. Larvae feed on fruit flesh and burrow into the seed, producing large amounts of frass and causing the fruits to drop from the tree prematurely. Larval damage renders the fruits unfit for commercial sale, leading to significant economic losses. The avocado seed moth has only been recorded as feeding on members of the Lauraceae family, with Persea americana (avocado) as the major host and other secondary hosts: P. schiedeana (coyo), wild Persea spp., and Beilschmedia spp. California accounts for the majority of avocado production in the U.S., followed by Florida and Hawaii. Stenoma catenifer is a small moth with few distinguishing features Fig. 1. Dorsal (top) and ventral (bottom) as an adult. -

Pest and Diseases in Mango (Mangifera Indica L.) J
PEST AND DISEASES IN MANGO (MANGIFERA INDICA L.) J. González-Fernández, J.I. Hormaza IHSM la Mayora CSIC-UMA, 29750 Algarrobo, Malaga, Spain EXECUTIVE SUMMARY In this work, we review the most important pests and diseases that affect mango production worldwide as well as the main measures implemented to control them. Pests and diseases are the main factors that can impact sustainable mango fruit production in the tropics and subtropics worldwide. Commercial cultivation of mango, characterized by expansion to new areas, changing crop management, replacement of varieties and increased chemical interventions, has altered significantly the pest and disease community structure in this crop in the different mango producing regions. In addition, climate change is inducing the emergence of new pests and, whereas globalization and trade liberalization have created wide opportunities for mango commercialization growth, at the same time, this can result in faster dispersion of pests and diseases among different mango growing areas if proper sanitary measures are not implemented. This review covers different topics related to pests and diseases in mango. First, a thorough description of the main pests and diseases that affect mango is provided. Second, the different approaches used in different mango producing countries for chemical and biological control are described. Third, recommendations for appropriate mango management techiques that include integrated pest and disease management, reduction in the use of chemicals and the implementation of a good monitoring and surveillance system to help control the main pests and diseases, are also discussed. Finally, the current knowledge on agrohomeopathy and Korean Natural Farming is analyzed and recommendations on future lines of research to optimize mango pest and disease control are discussed. -

Final Report
Final Report Coordination of Banana Industry R&D (Panama TR4) Project leader: Jim Pekin Delivery partner: Australian Banana Growers’ Council Project code: BA14012 Hort Innovation – Final Report Project: Coordination of Banana Industry R&D (Panama TR4) – BA14012 Disclaimer: Horticulture Innovation Australia Limited (Hort Innovation) makes no representations and expressly disclaims all warranties (to the extent permitted by law) about the accuracy, completeness, or currency of information in this Final Report. Users of this Final Report should take independent action to confirm any information in this Final Report before relying on that information in any way. Reliance on any information provided by Hort Innovation is entirely at your own risk. Hort Innovation is not responsible for, and will not be liable for, any loss, damage, claim, expense, cost (including legal costs) or other liability arising in any way (including from Hort Innovation or any other person’s negligence or otherwise) from your use or non‐use of the Final Report or from reliance on information contained in the Final Report or that Hort Innovation provides to you by any other means. Funding statement: This project has been funded by Hort Innovation, using the banana research and development levy and contributions from the Australian Government. Hort Innovation is the grower‐owned, not‐for‐profit research and development corporation for Australian horticulture. Publishing details: ISBN 978 0 7341 4433 1 Published and distributed by: Hort Innovation Level 8 1 Chifley Square -
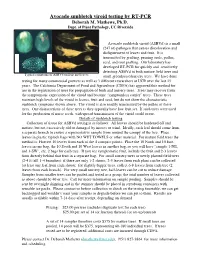
Avocado Sunblotch Viroid Testing by RT-PCR Deborah M
Avocado sunblotch viroid testing by RT-PCR Deborah M. Mathews, Ph.D. Dept. of Plant Pathology, UC Riverside Avocado sunblotch viroid (ASBVd) is a small (247 nt) pathogen that causes discoloration and disfigurement of leaves and fruit. It is transmitted by grafting, pruning tools, pollen, seed, and root grafting. Our laboratory has developed RT-PCR for quickly and sensitively detecting ASBVd in both mature field trees and Typical symptoms of ASBVd on fruit and leaves small greenhouse/nursery trees. We have done testing for many commercial growers as well as 3 different researchers at UCR over the last 15 years. The California Department of Food and Agriculture (CDFA) has approved this method for use in the registration of trees for propagation of buds and nursery trees. Trees may recover from the symptomatic expression of the viroid and become “symptomless carrier” trees. These trees maintain high levels of the viroid in leaves, fruit and seed, but do not show the characteristic sunblotch symptoms shown above. The viroid is also readily transmitted by the pollen of these trees. One characteristic of these trees is they typically have low fruit set. If such trees were used for the production of nurse seeds, widespread transmission of the viroid could occur. Details of sunblotch testing Collection of tissue for ASBVd testing is as follows: All leaves should be hardened off and mature, but not excessively old or damaged by insects or wind. Ideally, each leaf should come from a separate branch to ensure a representative sample from around the canopy of the tree. Place leaves in plastic ziplock bags with NO WET TOWELS or other material. -

A Molecular Approach to Studying Hymenoptera Diets Using Polistine Wasps
ORIG I NAL AR TI CLE P r e p r i n t A molecular approach to studying Hymenoptera diets using polistine wasps Lefort M.-C.1,2 | Beggs J.R.3 | Glare T.R.4 | Doyle E.J.2 | Saunders T.E.3 | Boyer S.5∗ 1Laboratoire d’Écologie et Biologie des Interactions (EBI) – UMR 7267 CNRS, The study of animal diets has benefited from the rise of Université de Poitiers, 5 rue Albert Turpain, high-throughput DNA sequencing applied to stomach con- 86073 POITIERS Cedex 9, France tent or faecal samples. The latter can be fresh samples used 2Environmental and Animal Sciences, to describe recent meals, or older samples, which can in- Unitec Institute of Technology, 139 form about past feeding activities. For most invertebrates, Carrington Road, Mt Albert, Auckland 1025, however, it is difficult to access ‘historical’ samples, due New Zealand to the small size of the animals and the absence of per- 3Centre for Biodiversity and Biosecurity, manent defecation sites. Therefore, sampling must be re- School of Biological Sciences|Te Kura Matauranga¯ Koiora, University of peated to account for seasonal variation and to capture the Auckland|Te Whare Wananga¯ o Tamaki¯ overall diet of a species. This study develops a method Makaurau, PB 92019 Auckland 1142, New to describe the overall diet of social Hymenoptera based Zealand on a single sampling event, by analysing prey DNA from 4Bio-Protection Research Centre, PO Box faeces accumulated in brood cells. We collected 48 nests 85084, Lincoln University, Lincoln 7647, from two species of introduced paper wasps (Polistes chi- Christchurch, New Zealand nensis, and P. -

The Tropical Fig Borer, Batocera Rufomaculata (Coleoptera: Cerambycidae), New for Turkey
The Tropical Fig Borer, Batocera rufomaculata (Coleoptera: Cerambycidae), new for Turkey by Göksel Tozlu and Hikmet Özbek Abstract: The Tropical Fig Borer, Batocera rufomaculata (De Geer, 1775) is recorded from the eastern Mediterranean Region of Turkey as a genus, species and a fig pest new for Turkey. The material collected and the views of some growers suggest that B. rufomaculata was probably in- troduced to Turkey from Israel, Lebanon, Syria or Iraq in the 1970s. Kurzfassung: Der Tropische Feigenbohrer, Batocera rufomaculata (De Geer, 1775) wird erst- mals aus der Türkei gemeldet, und zwar aus der östlichen Mittelmeeregion. Nicht nur die Art, auch der Genus ist neu für die Türkei; auch als Feigenschädling wird die Art in der Türkei erst- mals registriert. Aufgrund des gesammelten Materials und aufgrund von Berichten von Feigenan- bauern wird geschlossen, daß B. rufomaculata in die Türkei wahrscheinlich aus Israel, dem Liba- non, Syrien oder dem Irak eingeschleppt wurde. Key words: Batocera rufomaculata, Cerambycidae, fig borer, new record, new pest, alien spe- cies, Turkey, Middle East. Introduction The Tropical Fig Borer, Batocera rufomaculata (De Geer, 1775), has a tropical distribution, extending from southern China through Malaya, India, Sri Lanka, Madagascar and Mauritius to eastern Africa (AVIDOV & HARPAZ 1969, KATBEH-BADER 1996). This pest species was introduced into Israel in 1949 (AVIDOV & HARPAZ 1969) and into Jordan in the 1940s (KAT- BEH-BADER 1996). Following this, HEYROVSKY (1963) recorded the presence of this species in Jordan in 1957. Although HALPERIN & HOLZSCHUH (1993) indicated that B. rufomaculata has been disappearing in Israel since the 1970s, more recently KATBEH-BADER (pers. -

Detection of Avocado Sunblotch Viroid and Estimation of Infection Among Accessions in the National Germplasm Collection for Avocado
Proc. Fla. State Hort. Soc. 109:235-237. 1996. DETECTION OF AVOCADO SUNBLOTCH VIROID AND ESTIMATION OF INFECTION AMONG ACCESSIONS IN THE NATIONAL GERMPLASM COLLECTION FOR AVOCADO Catherine M. Running and Raymond J. Schnell National Germplasm Repository U. S. Department of Agriculture Agricultural Research Service 13601 Old Cutler Road, Miami, FL 33158 David N. Kuhn Florida International University Dept. of Biological Sciences Miami, FL 33199 Additional index words. Persea Americana, RT-PCR, viroid, DNA, RNA, sequence variation, germplasm. ABSTRACT Reverse transcription-polymerase chain reaction (RTPCR) was used to determine the incidence of infection by avocado sunblotch viroid (ASBVd) in the germplasm collection at the National Germplasm Repository at Miami (NGR-Mia). Of the 429 avocado plants growing at the repository, 81 (18.9%) are infected with the viroid. The 429 plants represent 237 accessions. There are multiple plants of some accessions and for 42 accessions (17%) every plant is infected with the viroid. There was no apparent relationship between host race and the incidence of infection. Symptoms of sunblotch disease on avocado (Persea Americana Mill.) manifest as a general decline of tree vigor with sunken, yellow areas on the fruit that lessen its marketability. The disease was first described as infectious, rather than physiological, by Home and Parker (1931). The infectious agent has since been determined to be an RNA viroid known as Avocado Sunblotch Viroid (ASBVd) (Dale and Allen, 1979; Thomas and Mohammed, 1979). The viroid is transmitted by budding and grafting, including natural root grafting (Home etal, 1941; Whitsell, 1952), seed (Wallace and Drake, 1962), and pollen (Desjardins et al., 1979).