BLOOD RESEARCH September 2020 ARTICLE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
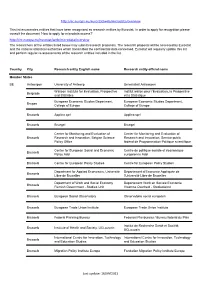
Eurostat: Recognized Research Entity
http://ec.europa.eu/eurostat/web/microdata/overview This list enumerates entities that have been recognised as research entities by Eurostat. In order to apply for recognition please consult the document 'How to apply for microdata access?' http://ec.europa.eu/eurostat/web/microdata/overview The researchers of the entities listed below may submit research proposals. The research proposal will be assessed by Eurostat and the national statistical authorities which transmitted the confidential data concerned. Eurostat will regularly update this list and perform regular re-assessments of the research entities included in the list. Country City Research entity English name Research entity official name Member States BE Antwerpen University of Antwerp Universiteit Antwerpen Walloon Institute for Evaluation, Prospective Institut wallon pour l'Evaluation, la Prospective Belgrade and Statistics et la Statistique European Economic Studies Department, European Economic Studies Department, Bruges College of Europe College of Europe Brussels Applica sprl Applica sprl Brussels Bruegel Bruegel Center for Monitoring and Evaluation of Center for Monitoring and Evaluation of Brussels Research and Innovation, Belgian Science Research and Innovation, Service public Policy Office fédéral de Programmation Politique scientifique Centre for European Social and Economic Centre de politique sociale et économique Brussels Policy Asbl européenne Asbl Brussels Centre for European Policy Studies Centre for European Policy Studies Department for Applied Economics, -

Demos / Posters
Demos / Posters The map of the rooms is available here DEMO SESSION 1 Tuesday, April 16 (10:30 - 16:00) Room: Corridoio Nizza Move-with-the-Sun or Move-with-the-Moon? Wide-area CloudNet Migrations Under Latency and Resource Constraints 1 / 14 Demos / Posters Johannes Grassler (TU Berlin & T-Labs, Germany); Stefan Schmid (T-Labs & TU Berlin, Germany); Anja Feldmann (TU-Berlin & Deutsche Telekom Laboratories, Germany) OPRSFEC: A Middleware of Packet Loss Recovery in Live Multicast Smart TV System Xiuyan Jiang (Fudan University, P.R. China); De jian Ye (Fudan University, P.R. China); Yiming Chen (Fudan University, P.R. China) Lossless Virtual Networks Daniel Crisan (ETHZ & IBM Research, Switzerland); Robert Birke (IBM Zurich Research Laboratory, Switzerland); Cyriel Minkenberg (IBM Research - Zurich, Switzerland); Mitchell Gusat (IBM Zurich research laboratory, Switzerland) Live Evaluation of Quality of Experience for Video Streaming and Web Browsing Louis Plissonneau (Orange Labs, France); Heng Cui (EURECOM, France); Ernst W Biersack (EURECOM, France) A User-centric Approach to Trust Management in Wi-Fi Networks Carlos Ballester (University of Geneva, Switzerland); Jean-Marc Seigneur (University of Geneva, Switzerland); Paolo Di Francesco (Level7, Italy); Valentin Moreno (FON, Spain); Rute C. Sofia (SITI, Universidade Lusófona, Portugal); Waldir Moreira (SITILabs, University Lusofona, Portugal); Alessandro Bogliolo (University of Urbino, Italy); Nuno Martins (Caixa Mágica Software, Portugal) User-centric Mobile Multimedia Service Delivery: -

Emidio Capriotti Phd CURRICULUM VITÆ
Emidio Capriotti PhD CURRICULUM VITÆ Name: Emidio Capriotti Nationality: Italian Date of birth: February, 1973 Place of birth: Roma, Italy Languages: Italian, English, Spanish Positions Oct 2019 Associate Professor: Department of Pharmacy and Biotechnology (FaBiT). University of Bologna, Bologna, Italy 2016-2019 Senior Assistant Professor (RTD type B): Department of Pharmacy and Biotechnology (FaBiT) and Department of Biological, Geological, and Environmental Sciences (BiGeA). University of Bologna, Bologna, Italy. 2015-2016 Junior Group Leader: Institute of Mathematical Modeling of Biological Systems, University of Düsseldorf, Düsseldorf, Germany 2012-2015 Assistant Professor: Division of Informatics, Department of Pathology, University of Alabama at Birmingham (UAB), Birmingham (AL), USA. 2011-2012 Marie-Curie IOF: Contracted Researcher at the Department of Mathematics and Computer Science, University of Balearic Islands (UIB), Palma de Mallorca, Spain. 2009-2011 Marie-Curie IOF: Postdoctoral Researcher at the Helix Group, Department of Bioengineering, Stanford University, Stanford (CA), USA. 2006-2009 Postdoctoral Researcher in the Structural Genomics Group at Department of Bioinformatics and Genetics, Prince Felipe Research Center (CIPF) Valencia, Spain. 2004-2006 Contract researcher at Department of Biology, University of Bologna, Bologna, Italy. 2001-2003 Ph.D student in Physical Sciences at University of Bologna, Bologna, Italy. Education Sep 2004 Master in Bioinformatics (first level) University of Bologna, Bologna (Italy). Jun 2004 Ph.D. in Physical Sciences University of Bologna, Bologna (Italy). Jul 1999 Laurea (B.S.) Degree in Physical Sciences, score 106/110 University of Bologna, Bologna (Italy). Visiting Jun 2012 – Jul 2012 Prof. Frederic Rousseau and Prof. Joost Schymkowitz, VIB Switch Laboratory, KU Leuven, Leuven (Belgium) May 2009 Prof. -

Curriculum Vitae Carlo Filippi
Curriculum Vitae Carlo Filippi Office address and URLs Department of Economics and Management - University of Brescia Contrada S. Chiara 50 - 25122 BRESCIA. Tel: +39 - 030 298 8576 E-mail: [email protected] OR group: http://or-brescia.unibs.it/ Scopus: https://www.scopus.com/authid/detail.uri?authorId=7006884051 Publons: https://publons.com/researcher/532100/carlo-filippi Google Scholar: http://scholar.google.it/citations?user=Wrwp_L0AAAAJ&hl=it CURRENT POSITION Associate Professor of Operations Research (MAT / 09) National Scientific Qualification, Full Professor, Operations Research. PROFESSIONAL EXPERIENCE 01/09/2006 – to the current date: Associate Professor of Operations Research (MAT / 09) University of Brescia - Department of Economics and Management, Brescia (Italy) Teaching: Currently Professor of Introduction to Logistics (6CFU, Laurea Master's Degree in Management), Supply Chain Management (6 CFU, Master's Degree in Management), Business Decision Analysis - AG (6 CFU, Bachelor's Degrees in Economics). Previously, professor and substitute teacher of various courses on logistics topics and decision support methods for master's degrees and on basic computer science topics and optimization for three-year degrees. Supervisor over 50 final reports for three-year and master's degrees in Economics, on topics related to logistics, optimization, information systems, electronic marketing. Research: Production of articles in international scientific journals and presentations at national and international sector conferences on topics -

(Brachytherapy) Education in Italy: Results of the INTERACTS Survey
Review Papers Review paper Current state of interventional radiotherapy (brachytherapy) education in Italy: results of the INTERACTS survey Luca Tagliaferri, MD, PhD1, György Kovács, MD, PhD2, Cynthia Aristei, MD3, Vitaliana De Sanctis, MD4, Fernando Barbera, MD5, Alessio Giuseppe Morganti, MD6, Calogero Casà, MD7, Bradley Rumwell Pieters, MD, PhD8, Elvio Russi, MD9, Lorenzo Livi, MD10, Renzo Corvò, MD11, Andrea Giovagnoni, MD12, Umberto Ricardi, MD13, Vincenzo Valentini, MD14,15, Stefano Maria Magrini, MD16 and the Directors of the Italian Radiation Oncology Schools** 1Chair of the Brachytherapy, Interventional Radiotherapy and IORT Study Group of the Italian Radiotherapy and Clinical Oncology Society (AIRO); Gemelli ART (Advanced Radiation Therapy), UOC Radioterapia, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy, 2INTERACTS (Interventional Radiotherapy Active Teaching School) Educational Program Director; Head, Interdisciplinary Brachytherapy Unit, University of Lübeck/UKSH CL, Lübeck, Germany, 3Past Chair of AIRO Brachytherapy study group; Head, Radiation Oncology Section, Department of Surgery and Biomedical Sciences, University of Perugia and Perugia General Hospital, Perugia, Italy, 4Deputy Chair of the Brachytherapy, Interventional Radiotherapy and IORT AIRO Study Group; Department of Radiation Oncology, Faculty of Medicina e Psicologia, Sant’Andrea Hospital, University of Rome “La Sapienza”, Rome, Italy, 5Board Member of the Brachytherapy, Interventional Radiotherapy and IORT AIRO Study Group; Head, Brachytherapy -

Dr Benedicta Marzinotto
CURRICULUM VITAE BENEDICTA MARZINOTTO Contact details: Rue de la Charité 33, B-1210 Brussels, Belgium [email protected] CURRENT EMPLOYMENT 2010- Research fellow, Bruegel, Brussels, Belgium 2005- Lecturer in Political Economy, Economics Department, University of Udine, Italy EDUCATION 2005 PhD in European Political Economy, London School of Economics (LSE), UK 2002 MPhil in European Political Economy, LSE, UK 1999 MSc in European Studies, LSE, UK RESEARCH INTERESTS European economic governance; political economy of fiscal adjustment; central banks and wage bargaining systems; varieties of capitalism; international trade and New Keynesian models. FELLOWSHIPS, HONOURS AND GRANTS 2005-10 Associate Fellow, International Economics, Chatham House, London, UK 2008 Visiting Scholar, University of Auckland, New Zealand 2007 Visiting Fellow, DGECFIN, European Commission, Brussels, Belgium 2005-07 Academic Collaboration – International Network Grant, Leverhulme Trust 2000-05 LSE Research Studentship 2004 Visiting Research Associate, Free University of Berlin, Germany 2004 UACES Research Scholarship 2002 Visiting Researcher, European University Institute, Fiesole, Italy TEACHING EXPERIENCE 2011- “Monetary Policy”, University of Udine, Italy 2009- “EU Macroeconomic Policy and Economic Governance”, College of Europe, Natolin Campus, Warsaw, Poland 2005- “Macroeconomics”, University of Udine, Italy 1 2006-08 “Government and Policies of the European Union”, MSc Mercosur and the EU in Comparative Perspective, Universidad Nacional de Cuyo, -

International Incoming Student's Guide
INTERNATIONAL INCOMING STUDENT’S GUIDE What you need to get the most out of Pavia and its university, and survive! UNIVERSITÀ DEGLI STUDI DI PAVIA Corso Strada Nuova 65, 27100 Pavia, Italy e-mail: [email protected] Tel. +39 0382 984021 Fax +39 0382 984695 Index 1. INTRODUCTION 9 1.1 THE INTERNATIONAL RELATIONS OFFICE AND WELCOME POINT 9 1.2 THE ITALIAN ACADEMIC SYSTEM 9 1.3 FACULTIES 10 1.4 PLACES YOU NEED FOR YOUR STUDIES 11 2. MEANS OF TRANSPORT 12 2.1 AIRPLANES AND AIRPORTS GETTING TO PAVIA 12 2.2 TRAINS AND STATIONS 12 2.2.1 TICKET BIGLIETTO 12 2.3 BUSES AND COACHES AUTOBUS E CORRIERE 13 3. BUREAUCRACY 15 3.1 LOST PASSPORT 15 4. UNIVERSITY OF PAVIA 16 4.1 SERVICES 16 4.1.1 CANTEENS MENSA 16 4.1.2 COMPUTER FACILITIES AND WI-FI CONNECTION 17 4.1.3 BOOKS LIBRI 17 4.1.4 LIBRARIES AND OPAC BIBLIOTECHE 17 4.1.5 BOOKSTORES LIBRERIE 18 4.2 UNIVERSITY LIFE VITA UNIVERSITARIA 19 4.2.1 C.U.S. UNIVERSITY SPORTS CENTRE 19 4.2.2 S.A.I.S.D. FACILITIES FOR DISABLED STUDENTS 19 4.2.3 STUDENT ASSOCIATIONS 19 4.2.4 MERCHANDISING 20 4.2.5 UCAMPUS 20 5. HEALTH 21 5.1 HEALTH INSURANCE ASSICURAZIONE SANITARIA 21 5.2 FIRST AID PRONTO SOCCORSO 21 5.3 PHARMACIES FARMACIA 22 5.4 MEDICINES MEDICINALI 23 6. FOOD AND FUN 25 6.1 SUPERMARKETS AND MARKET PLACES 25 6.2 BARS, PIZZERIAS AND RESTAURANTS 26 6.3 NIGHTLIFE - WHAT DOES PAVIA OFFER AT NIGHT? 27 7. -

Midwifery Education Institutions in Italy Creation and Validation of Clinical Preceptors’ Assessment Tool: Students’ and Expert Midwives’ Views
Article Midwifery Education Institutions in Italy Creation and Validation of Clinical Preceptors’ Assessment Tool: Students’ and Expert Midwives’ Views Paola Agnese Mauri 1,2,* , Ivan Cortinovis 1 , Norma Nilde Guerrini Contini 2 and Marta Soldi 2 1 Department of Clinical Sciences and Community Health, Università degli Studi di Milan, via Manfredo Fanti 6, 20122 Milan, Italy; [email protected] 2 Unit of Mother Child and Newborn Health, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, via Manfredo Fanti 6, 20122 Milan, Italy; [email protected] (N.N.G.C.); [email protected] (M.S.) * Correspondence: [email protected]; Tel./Fax: +39-025-503-8599 Received: 20 November 2020; Accepted: 14 December 2020; Published: 16 December 2020 Abstract: Background: The aim of the study is to create and validate a midwifery preceptor’s evaluation form to be used by midwifery students. The International Confederation of Midwives recommends that clinical placements need to be supervised by a preceptor in order to be efficient for students who, in this way, gain competence and proper practice within the midwifery practical area. Methods: This is an observational multi-center transversal study and leads to the validation of an evaluation questionnaire. Methodically, the following steps were followed: literature review, focus group with midwifery students, meeting between expert midwives, creation of the preceptor’s assessment form, filling in of the forms by midwifery students and expert midwives, and validation of the form. The study was carried out in eight Italian universities and included eighty-eight midwifery students and eight midwives. Results and Conclusion: A midwifery preceptor’s assessment questionnaire was created made up of four attribute areas which, as a total, included 33 items. -

Appendix 2 Co-Investigators Name Location Role Contribution Patrizia Accorsi ASST Spedali Civili of Brescia, Italy Site Investi
Appendix 2 Co-investigators Name Location Role Contribution ASST Spedali Civili of Site Participant Patrizia Accorsi Brescia, Italy investigator recruitment/follow-up "V. Buzzi" Children's Site Participant Enrico Alfei Hospital, University of Milan, investigator recruitment/follow-up Italy Meyer Children's University Site Participant Elena Andreucci Hospital, Florence, Italy investigator recruitment/follow-up Fondazione IRCCS Ca' Gianluigi Site Participant Granda, Ospedale Maggiore Ardissino investigator recruitment/follow-up Policlinico, Milan, Italy Oasi Research Institute- Site Participant Emanuela Avola IRCCS, Troina, Italy investigator recruitment/follow-up University of Catania; CNR, Site Participant Rita Barone Catania, Italy investigator recruitment/follow-up Francesco Regional Hospital of Bolzano, Site Participant Benedicenti Italy investigator recruitment/follow-up University Hospital of Ferrara, Site Participant Stefania Bigoni Italy investigator recruitment/follow-up Microcitemie Regional Loredana Site Participant Hospital, Brotzu Hospital, Boccone investigator recruitment/follow-up Cagliari IRCCS Istituto Auxologico Site Participant Maria T. Bonati Italiano, Milan, Italy investigator recruitment/follow-up "V. Buzzi" Children's Site Participant Stefania Bova Hospital, University of Milan, investigator recruitment/follow-up2 Italy Marilena Site Participant University of Messina, Italy Briguglio investigator recruitment/follow-up Site Participant Silvana Briuglia University of Messina, Italy investigator recruitment/follow-up -

Freshwater and Culture Water Resources Management and Culture
INTERNATIONAL CONFERENCE Freshwater and Culture Water Resources Management and Culture Padiglione KIP Interna@onal School - EXPO Milano 2015 October 6-7 2015 Comune di Tivoli Organized by With the contribuon of With the patronage of PROGRAMME 10:00 Welcome Gree@ngs 14:30 Session I: Cultural Heritage Luciano Carrino KIP Internaonal School President Co-Chairs 10:30 Opening Speeches Ruggero Mar@nes Tivoli Municipality, Assessore Silvano RomeX Umbria Region University for Foreigners Perugia, Rector Giovanni Paciullo Fabio Paparelli Regione Umbria Vice President Invited Speakers: Ruggero Mar@nes Tivoli Municipality, Assessore Maurizio Memo University of Brescia, Deputy Rector Baldassarre Bacchi University of Brescia Gabriella Zanferrari Ital ICID President Fabio Bianconi University of Perugia Alessandro Folli URBIM Lombardia, President Chiara Biscarini University for Foreigners Perugia Gianluca Boer University of Padova 11:00 Lec@o Magistralis Franco Cotana University of Perugia Claudio Gandolfi University of Milano Angelo Cavallin University of Milan Bicocca Salvatore Grimaldi Tuscia University Cairoli Giuliani Emerito Sapienza University of Rome Francesco Laio Polytechnic of Turin Enzo Grossi EXPO 2015 Scienfic Advisor Italian Pavillon Paolo Mignosa University of Parma Luigi Natale University of Pavia Fernando Nardi University for Foreigners Perugia Ugo Ruffolo University of Bologna Donatella Padua University for Foreigners Perugia Mario Torelli Accademia dei Lincei Elena Ridolfi University of Perugia Stefano Sibilla University of Pavia 20:00 -

AAMAS 2019 Program Committee
AAMAS 2019 Programme Committee Diana Francisca Adamatti, Universidade Federal do Rio Johan Barthelemy, University of Wollongong - SMART, Grande, Brazil Australia Adrian Agogino, NASA Ames Research Center, USA Nicola Basilico, University of Milan, Italy Pritee Agrawal, Singapore Management University, Rahmatollah Beheshti, University of Delaware, USA Singapore Xiaohui Bei, Nanyang Technological University, Aamir Ahmad, Max Planck Institute for Intelligent Singapore Systems, Germany Jonathan Ben-Naim, CNRS, University of Toulouse, Reza Ahmadzadeh, University of Massachusetts - IRIT, France Lowell, USA Nawal Benabbou, Sorbonne Université - LIP6, France Nirav Ajmeri, North Carolina State University, USA Jonas Beskow, KTH, Sweden Charilaos Akasiadis, Technical University of Crete, Graeme Best, Oregon State University, USA Greece Elisabetta Bevacqua, Lab-STICC, ENIB, France Mohammad Al-Zinati, Jordan University of Science and Floris Bex, Utrecht University & University of Tilburg, Technology, Jordan Netherlands Dario Albani, University of Rome "La Sapienza", Italy Aurélie Beynier, LIP6, Sorbonne Université, France Michael Albert, University of Virginia, USA Georgios Birmpas, University of Oxford, UK Stefano Albrecht, The University of Edinburgh, UK Péter Biró, Hungarian Academy of Sciences, Hungary Shani Alkoby, The University of Texas at Austin, USA Filippo Bistaffa, IIIA-CSIC, Spain Shaull Almagor, University of Oxford, UK Stefano Bistarelli, Università degli Studi di Perugia, Javier Alonso-Mora, Delft University of Technology, Italy Netherlands -

Invest Your Talent in Italy Our Mission
3000 51 DAILY # Euros thrown into the Trevi fountain of ROME. #UNESCO WORLD HERITAGE SITES Donated to Charity MORE THAN ANY OTHER COUNTRY IN THE WORLD 48M 13 VISITORS YEARLY Fifth most visited country in the world Italians have won more Oscars (BEST FOREIGN FILM) than any other Country 1088 8th University of BOLOGNA FOUNDED Oldest European University Largest Economy in the world PARTNERS INSTITUTIONS Ministry of Foreign Affairs and International Cooperation UNIVERSITIES Italian Trade Agency Uni – Italia Unioncamere COMPANIES Confindustria INVEST YOUR TALENT IN ITALY OUR MISSION •Master’s and Postgraduate courses in Italy •On-the-job training at leading Italian companies (while completing your academic work) •Scholarships and student support services Entry qualification required: 3 or 4 years bachelor’s degree WHAT YOU STUDY 2016 COURSES ECONOMICS/ MANAGEMENT ENGINEERING/ICT 53 66 Courses Courses DESIGN/ ALL COURSES ARE IN ENGLISH ACHITECTURE At top Italian Universities 11 Courses ATTEND SOME OF THE BEST ITALIAN UNIVERSITIES POST GRADUATE COURSES 18 CITIES 130 Courses ATTEND SOME OF THE BEST ITALIAN UNIVERSITIES POST GRADUATE COURSES UNIVERSITY OF BRESCIA UNIVERSITY OF CALABRIA 22 UNIVERSITY OF FERRARA UNIVERSITY OF FIRENZE Italian UNIVERSITY OF GENOVA Universities UNIVERSITY OF SALENTO UNIVERSITY OF MACERATA IULM - INTERNATIONAL UNIVERSITY OF LANGUAGES AND MEDIA UNIVERSITY OF MILANO “BICOCCA” POLITECNICO DI MILANO UNIVERSITY OF MODENA AND REGGIO UNIVERSITY OF PADOVA UNIVERSITY OF PAVIA UNIVERSITY FOR FOREIGNERS “DANTE ALIGHIERI” LINK CAMPUS UNIVERSITY LUISS - Guido Carli Free International University for Social Studies UNIVERSITY OF ROMA “LA SAPIENZA” UNIVERSITY OF ROMA “TOR VERGATA” POLITECNICO DI TORINO UNIVERSITY OF TRENTO UNIVERSITY OF UDINE UNIVERSITY OF VERONA WAYS WE SUPPORT YOU INVEST YOUR TALENT IN ITALY 8000 EURO + Tuition HELPING HAND fee exemption Universities have desks to help find accommodations, integrate LANGUAGE qualified applicants will receive INTERNSHIPS scholarship funds into city etc.