Butyl Ether by a Filamentous Fungus, a Graphium Sp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Formation of Butane in the Polymerization of Methyl Methacrylate with Butyllithium
Polymer Journal, Vol. 12, No. 8, pp 535-537 (1980) SHORT COMMUNICATION The Formation of Butane in the Polymerization of Methyl Methacrylate with Butyllithium Koichi HATADA, Tatsuki KITAYAMA, and Heimei YUKI Department of Chemistry, Faculty of Engineering Science, Osaka University, Machikaneyama, Toyonaka, Osaka 560, Japan. (Received February 28, 1980) KEY WORDS Methyl Methacrylate I Perdeuterated Methyl Methacrylate I Anionic Polymerization I Butyllithium I Toluene I Tetrahydrofuran I Butane Formation I Mass Spectroscopy I Butene I It has been found that a small amount of butane merization reactions in toluene: (1) the poly is formed when the polymerization of methyl merization of MMA terminated by CH30D methacrylate is initiated with butyllithium and (MMA-CH30D system) and (2) that of MMA-d8 terminated with acetic acid. The origin of the butane terminated by CH3 0H (MMA-d8-CH3 0H system). was not clear, although the proton abstraction from The reactions were quenched at 10 min following the monomer is assumed. 1 the initiation. The spectra of butane (BuH) and In this work the polymerizations of undeuterated butane-d-1, CH3 CH2 CH2CH2 D, (BuD) are also (MMA) and perdeuterated (MMA-d8 ) methyl shown for comparison. The BuH and BuD were methacrylates were initiated with undeuterated obtained by the reaction of BuLi with CH30H and butyllithium (BuLi) and terminated by CH3 0D and CH30D, respectively. By inspecting these four CH3 0H, respectively. Deuterium distributions in spectra, it can be seen that the butane from the the butane formed were determined by combined MMA-CH3 0D system mainly consists of BuD and gas-liquid phase chromatography-mass spectrom the butane from the MMA-d8-CH30H system is etry. -

Safety Data Sheet
SAFETY DATA SHEET 1. Identification Product number 1000007900 Product identifier AUTO GLASS QUICK RELEASE AGENT Company information Sprayway, Inc. 1005 S. Westgate Drive Addison, IL 60101 United States Company phone General Assistance 1-630-628-3000 Emergency telephone US 1-866-836-8855 Emergency telephone outside 1-952-852-4646 US Version # 01 Recommended use Not available. Recommended restrictions None known. 2. Hazard(s) identification Physical hazards Flammable aerosols Category 1 Health hazards Serious eye damage/eye irritation Category 2A Specific target organ toxicity, single exposure Category 3 narcotic effects Aspiration hazard Category 1 Environmental hazards Not classified. OSHA defined hazards Not classified. Label elements Signal word Danger Hazard statement Extremely flammable aerosol. May be fatal if swallowed and enters airways. Causes serious eye irritation. May cause drowsiness or dizziness. Precautionary statement Prevention Keep away from heat/sparks/open flames/hot surfaces. - No smoking. Do not spray on an open flame or other ignition source. Pressurized container: Do not pierce or burn, even after use. Avoid breathing gas. Wash thoroughly after handling. Use only outdoors or in a well-ventilated area. Wear eye/face protection. Response If swallowed: Immediately call a poison center/doctor. If inhaled: Remove person to fresh air and keep comfortable for breathing. If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Call a poison center/doctor if you feel unwell. Do NOT induce vomiting. If eye irritation persists: Get medical advice/attention. Storage Store in a well-ventilated place. Keep container tightly closed. Store locked up. -

Site-Directed Amino Acid Substitutions in the Hydroxylase
JOURNAL OF BACTERIOLOGY, July 2006, p. 4962–4969 Vol. 188, No. 13 0021-9193/06/$08.00ϩ0 doi:10.1128/JB.00280-06 Copyright © 2006, American Society for Microbiology. All Rights Reserved. Site-Directed Amino Acid Substitutions in the Hydroxylase ␣ Subunit of Butane Monooxygenase from Pseudomonas butanovora: Implications for Substrates Knocking at the Gate Kimberly H. Halsey,1 Luis A. Sayavedra-Soto,2 Peter J. Bottomley,3 and Daniel J. Arp2* Molecular and Cellular Biology Program,1 Department of Botany and Plant Pathology,2 Department of Microbiology,3 Oregon State University, Cordley 2082, Corvallis, Oregon 97331-2902 Received 22 February 2006/Accepted 22 April 2006 Butane monooxygenase (BMO) from Pseudomonas butanovora has high homology to soluble methane mono- oxygenase (sMMO), and both oxidize a wide range of hydrocarbons; yet previous studies have not demon- strated methane oxidation by BMO. Studies to understand the basis for this difference were initiated by making single-amino-acid substitutions in the hydroxylase ␣ subunit of butane monooxygenase (BMOH-␣)inP. butanovora. Residues likely to be within hydrophobic cavities, adjacent to the diiron center, and on the surface of BMOH-␣ were altered to the corresponding residues from the ␣ subunit of sMMO. In vivo studies of five site-directed mutants were carried out to initiate mechanistic investigations of BMO. Growth rates of mutant strains G113N and L279F on butane were dramatically slower than the rate seen with the control P. butanovora wild-type strain (Rev WT). The specific activities of BMO in these strains were sevenfold lower than those of Downloaded from Rev WT. Strains G113N and L279F also showed 277- and 5.5-fold increases in the ratio of the rates of 2-butanol production to 1-butanol production compared to Rev WT. -

Aerobic Cometabolism of Chlorinated Aliphatic Hydrocarbons by Subsurface Microbes Grown on Methane, Propane and Butane from the Mcclellan Air Force Base
AN ABSTRACT OF THE THESIS OF Adisorn Tovanabootr for the degree of Masters of Science in Civil Engineering presented on April 23, 1997. Title: Aerobic Cometabolism of Chlorinated Aliphatic Hydrocarbons by Subsurface Microbes Grown on Methane, Propane and Butane from the McClellan Air Force Base. Abstract approved: Lewis Semprini Subsurface microorganisms from the McClellan Air Force Base were grown in batch aquifer microcosms on methane, propane, and butane as gaseous cometabolic substrates. The potential for aerobic cometabolism of chlorinated aliphatic hydrocarbons including trichloroethylene (TCE), 1,1,1-trichloroethane (1,1,1-TCA), and chloroform (CF) was determined. Stimulation of microorganisms on all of the substrates tested indicates a diverse microbial community exists in the McClellan subsurface. Indigenous methane and propane-utilizers were capable of transforming TCE and CF. Propane- utilizers very effectively transformed TCA, while methane-utilizers could not transform 1,1,1-TCA. The butane-utilizers were not able to degrade any of the CAHs tested. TCE was transformed most rapidly during the period of active methane consumption, and continued at a slower rate for about 1 weeks after methane was consumed. The propane culture remained active for up to four weeks after propane was consumed, and the rate followed first order kinetics. Different TCE transformation yields (Tv) (mg CAH/mg substrate) developed in replicate microcosms with time. Changes in TCE transformation ability resulted from changes in TCE concentration or TCE product toxicity. Both methane and propane-utilizers showed a positive correlation between initial TCE transformation rates and primary substrate utilization rates. The ratio of the zero order TCE transformation rate to primary substrate utilization rate was directly proportional to the ultimate transformation yield. -
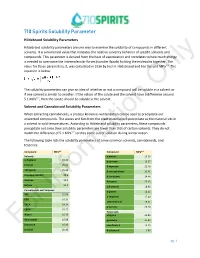
710 Spirits Solubility Parameter Hildebrand Solubility Parameters
710 Spirits Solubility Parameter Hildebrand Solubility Parameters Hildebrand solubility parameters are one way to examine the solubility of compounds in different solvents. It is a numerical value that indicates the relative solvency behavior of specific solvents and compounds. This parameter is derived from the heat of vaporization and correlates to how much energy is needed to overcome the intermolecular forces (van der Waals) holding the molecules together. The value for these parameters, δ, was calculated in 1936 by Joel H. Hildebrand and has the unit MPa1/2. The equation is below: The solubility parameters can give an idea of whether or not a compound will be soluble inOnly a solvent or if one solvent is similar to another. If the values of the solute and the solvent have a difference around 5.1 MPa1/2, then the solute should be soluble in the solvent. Solvent and Cannabinoid Solubility Parameters When extracting cannabinoids, a process known as winterization can be used to precipitate out unwanted compounds. The waxes and fats from the plant material will precipitate as the material sits in a solvent in cold temperatures. According to Hildebrand solubility parameters, these compounds precipitate out since their solubility parameters are lower than that of certain solvents. They do not match the difference of 5.1 MPa1/2 so they come out of solution during winterization. The following table lists the solubility parameters of some common solvents, cannabinoids, and terpenes: Compound MPa1/2 Compound MPa1/2 Solvents a-pinene 16.16 n-Heptane 15.40 p-cymene 16.57 Ethanol 26.20 B-myrcene 21.46 710 Spirits 25.66 B-caryophyllene 21.41 Isopropyl Alcohol 23.6 d-Limonene 24.46 Acetone 19.9 Pulegone 25.65 Butane 14.1 1,8-cineole 18.96 Cannabinoids and Terpenes a-pinene 16.15 THC Information22.09 a-terpineol 17.10 CBD 24.34 terpineol-4-ol 18.21 THCA 24.14 p-cymene 24.20 CBDA 25.77 Flavonoids Waxes 16.36 apigenin 20.80 Chlorophyll 16.98 quercetin 25.89 Limonene 16.90 cannflavin A 10.35 ForLinalool 20.46 B-sitosterol 3.86 pg. -

Technical Protocol for Evaluating Natural Attenuation of Chlorinated
United States Office of Research and EPA/600/R-98/128 Environmental Protection Development September 1998 Agency Washington DC 20460 Technical Protocol for Evaluating Natural Attenuation of Chlorinated Solvents in Ground Water TECHNICAL PROTOCOL FOR EVALUATING NATURAL ATTENUATION OF CHLORINATED SOLVENTS IN GROUND WATER by Todd H. Wiedemeier Parsons Engineering Science, Inc. Pasadena, California Matthew A. Swanson, David E. Moutoux, and E. Kinzie Gordon Parsons Engineering Science, Inc. Denver, Colorado John T. Wilson, Barbara H. Wilson, and Donald H. Kampbell United States Environmental Protection Agency National Risk Management Research Laboratory Subsurface Protection and Remediation Division Ada, Oklahoma Patrick E. Haas, Ross N. Miller and Jerry E. Hansen Air Force Center for Environmental Excellence Technology Transfer Division Brooks Air Force Base, Texas Francis H. Chapelle United States Geological Survey Columbia, South Carolina IAG #RW57936164 Project Officer John T. Wilson National Risk Management Research Laboratory Subsurface Protection and Remediation Division Ada, Oklahoma NATIONAL RISK MANAGEMENT RESEARCH LABORATORY OFFICE OF RESEARCH AND DEVELOPMENT U. S. ENVIRONMENTAL PROTECTION AGENCY CINCINNATI, OHIO 45268 i NOTICE The information in this document was developed through a collaboration between the U.S. EPA (Subsurface Protection and Remediation Division, National Risk Management Research Laboratory, Robert S. Kerr Environmental Research Center, Ada, Oklahoma [SPRD]) and the U.S. Air Force (U.S. Air Force Center for Environmental Excellence, Brooks Air Force Base, Texas [AFCEE]). EPA staff were primarily responsible for development of the conceptual framework for the approach presented in this document; staff of the U.S. Air Force and their contractors also provided substantive input. The U.S. Air Force was primarily responsible for field testing the approach presented in this document. -

Butane Propane
Safety Data Sheet Product: Butane-Propane Mixture Page: 1 of 6 Issued: 12-November-2015 SECTION 1 - IDENTIFICATION: PRODUCT IDENTIFIER AND CHEMICAL IDENTITY The Coleman Company, Inc. 3600 N Hydraulic Wichita, Kansas 67219 Phone: 1 800 835 3278 (bus hours) Chemical Nature: Petroleum Gases, Liquefied Trade Name: Butane-Propane Mixture Product Use: Disposable cartridges for use in portable gas appliances Creation Date: 13-March-2013 Revision Date: 12-November-2015 SECTION 2 - HAZARD IDENTIFICATION UN Number: 2037, Receptacles, small, containing gas (gas cartridges) flammable, without release device, not refillable and not exceeding 1 L capacity Classification: Flammable Gases, Category 1 SUSDP Classification: S6 Hazard DANGER Statements Extremely flammable gas. Harmful if inhaled. Causes skin irritation. Causes serious eye irritation. Precautionary Keep out of the reach of children. Statements Keep container in a well-ventilated place at a temperature not exceeding 48oC. Keep away from heat and ignition sources - No Smoking. In case of fire, use dry chemical, water fog or foam. Use only in well ventilated areas. Wash thoroughly with soap and water after handling and before eating, drinking or using tobacco. In case of insufficient ventilation, wear suitable respiratory equipment. Remove to fresh air immediately. Get medical attention immediately. Do NOT induce vomiting. Get immediate medical attention. If frostbite, call a physician. Hold eyelids apart and flush eyes with plenty of water for at least 15 minutes. Get medical attention. Avoid -

Safety Data Sheet
SAFETY DATA SHEET Flammable Gas Mixture: Acetone / Argon / C6+(Hexanes Plus) / Carbon Dioxide / Ethane / Ethanol / Helium / Hydrogen / Isobutane / Isopentane / Isopropyl Alcohol / Methane / N-Butane / N-Pentane / Neopentane / Nitrogen / Propane Section 1. Identification GHS product identifier : Flammable Gas Mixture: Acetone / Argon / C6+(Hexanes Plus) / Carbon Dioxide / Ethane / Ethanol / Helium / Hydrogen / Isobutane / Isopentane / Isopropyl Alcohol / Methane / N-Butane / N-Pentane / Neopentane / Nitrogen / Propane Other means of : Not available. identification Product use : Synthetic/Analytical chemistry. SDS # : 018560 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE GASES - Category 1 substance or mixture GASES UNDER PRESSURE - Compressed gas TOXIC TO REPRODUCTION (Fertility) - Category 2 TOXIC TO REPRODUCTION (Unborn child) - Category 2 AQUATIC HAZARD (LONG-TERM) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : Extremely flammable gas. Contains gas under pressure; may explode if heated. May form explosive mixtures in Air. May displace oxygen and cause rapid suffocation. May increase respiration and heart rate. Suspected of damaging fertility or the unborn child. Harmful to aquatic life with long lasting effects. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. -

Cometabolic Degradation of Polycyclic Aromatic Hydrocarbons (Pahs) and Aromatic Ethers by Phenol- and Ammonia-Oxidizing Bacteria
AN ABSTRACT OF THE DISSERTATION OF Soon Woong Chang for the degree of Doctor of Philosophy in Civil Engineering presented on November 11, 1997. Title: Cometabolic Degradation of Polycyclic Aromatic Hydrocarbons (PAHs) and Aromatic Ethers by Phenol- and Ammonia-Oxidizing Bacteria Redacted for Privacy Abstract approved: /Kenneth J. Williamson Cometabolic biodegradation processes are potentially useful for the bioremediation of hazardous waste sites. In this study the potential application of phenol- oxidizing and nitrifying bacteria as "priming biocatalysts" was examined in the degradation of polycyclic aromatic hydrocarbons (PAHs), aryl ethers, and aromatic ethers. We observed that a phenol-oxidizing Pseudomonas strain cometabolically degrades a range of 2- and 3-ringed PAHs. A sequencing batch reactor (SBR) was used to overcome the competitive effects between two substrates and the SBR was evaluated as a alternative technology to treat mixed contaminants including phenol and PAHs. We also have demonstrated that the nitrifying bacterium Nitrosomonas europaea can cometabolically degrade a wide range polycyclic aromatic hydrocarbons (PAHs), aryl ethers and aromatic ethers including naphthalene, acenaphthene, diphenyl ether, dibenzofuran, dibenzo-p-dioxin, and anisole. Our results indicated that all the compounds are transformed by N. europaea and that several unusual reactions are involved in these reactions. In the case of naphthalene oxidation, N. europaea generated predominantly 2 naphthol whereas other monooxygenases generate 1-naphthol as the major product. In the case of dibenzofuran oxidation, 3-hydroxydibenzofuran initially accumulated in the reaction medium and was then further transformed to 3-hydroxy nitrodibenzofuran in a pH- and nitrite-dependent abiotic reaction. A similar abiotic transformation reaction also was observed with other hydroxylated aryl ethers and PAHs. -

A Model of in Situ Bioremediation That Includes the Effect of Rate- Limited Sorption and Bioavailability J
A MODEL OF IN SITU BIOREMEDIATION THAT INCLUDES THE EFFECT OF RATE- LIMITED SORPTION AND BIOAVAILABILITY J. Huang and M.N. Goltz Department of Engineering and Environmental Management, Air Force Institute of Technol- ogy, Wright-Patterson Air Force Base, OH 45433-7765; Phone: (937) 255-2998, Fax: (937) 656-4699 ABSTRACT In situ bioremediation is a groundwater and soil remediation technology that makes use of indigenous or introduced microorganisms to degrade contaminants in the subsurface. One factor affecting the feasibility of in situ bioremediation is the availability of the contaminant to the microorganisms. It is typically assumed that the contaminant is only available for biodegradation in the aqueous phase. However, many organic contami- nants of interest are sorbed to soil in the subsurface, and the sorbed contaminant is presumably unavailable for bioremediation. If bioremediation is attempted, the rate of desorption of contaminant from the sorbed phase is a factor in determining the availability of the contaminant to the degrading microorganisms and hence, the efficacy of the remediation. In this work, a transport model is presented that accounts for both biodegradation and desorption kinetics. The model is run using realistic parameter values obtained from a recent field evaluation of in situ aerobic cometabolic bioremediation at Edwards AFB, to demonstrate the potential impact of rate-limited sorption on bioremediation efficacy. The model allows remediation managers to better understand the impact of the in situ bioremediation technology on the fate and transport of contaminants, ultimately helping these managers design and implement solutions to contamination problems. Key words: modeling, bioavailability, sorption, cometabolism, bioremediation BACKGROUND In situ bioremediation is a technology that is currently being studied (and increasingly imple- mented) in the hope that it may be useful in remediating the nations hazardous waste sites. -

Section 5. Fire-Fighting Measures Extinguishing Media Suitable Extinguishing : Use an Extinguishing Agent Suitable for the Surrounding Fire
SAFETY DATA SHEET Flammable Gas Mixture: 1-Butene / Acetylene / Carbon Monoxide / Ethane / Ethylene / Hydrogen / Isobutane / Methane / Methyl Acetylene / N-Butane / Propadiene / Propane / Propylene Section 1. Identification GHS product identifier : Flammable Gas Mixture: 1-Butene / Acetylene / Carbon Monoxide / Ethane / Ethylene / Hydrogen / Isobutane / Methane / Methyl Acetylene / N-Butane / Propadiene / Propane / Propylene Other means of : Not available. identification Product use : Synthetic/Analytical chemistry. SDS # : 012433 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 Emergency telephone : 1-866-734-3438 number (with hours of operation) Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE GASES - Category 1 substance or mixture GASES UNDER PRESSURE - Compressed gas SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Narcotic effects) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : Extremely flammable gas. Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. May cause drowsiness and dizziness. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Approach suspected leak area with caution. -

Butane-Commercial Grade
SAFETY DATA SHEET 1. Identification Product identifier Butane (Commercial Grade) Other means of identification SDS number 5 Recommended use Fuel. Recommended restrictions None known. Manufacturer / Importer / Supplier / Distributor information Company name DCP Midstream Address 370 17 Street Suite 2500 Denver, CO 80202 Telephone (303) 595-3331 E-mail [email protected] Contact person Mark Prewitt Emergency phone number CHEMTREC - 24 HOURS: 800-424-9300 2. Hazard(s) identification Physical hazards Flammable gases Category 1 Gases under pressure Liquefied gas Health hazards Not classified. OSHA hazard(s) Simple asphyxiant Label elements Hazard symbol Signal word Danger Hazard statement Extremely flammable gas. Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. Precautionary statement Prevention Keep away from heat/sparks/open flames/hot surfaces. - No smoking. Response Leaking gas fire: Do not extinguish, unless leak can be stopped safely. Eliminate all ignition sources if safe to do so. Storage Store in a well-ventilated place. Disposal Dispose of contents/container in accordance with local/regional/national/international regulations. Hazard(s) not otherwise Not classified. classified (HNOC) 3. Composition/information on ingredients Mixture Hazardous components Chemical name Common name and synonyms CAS number % Butane 106-97-8 94 Isobutane 75-28-5 4 Pentane 109-66-1 2 Propane 74-98-6 1 Butane (Commercial Grade) 1/ 9 91653 Version#: 01 Revision date: - 10-19-2016 Issue Date: 11-28-2012 Composition comments All concentrations are in percent by weight unless ingredient is a gas. Gas concentrations are in percent by volume. 4. First-aid measures Inhalation Immediately remove from further exposure.