NCCN Guidelines for Patients Diffuse Large B-Cell Lymphoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primary Mediastinal Large B-Cell Lymphoma
Primary Mediastinal Large B-Cell Lymphoma Page 1 of 13 Disclaimer: This algorithm has been developed for MD Anderson using a multidisciplinary approach considering circumstances particular to MD Anderson’s specific patient population, services and structure, and clinical information. This is not intended to replace the independent medical or professional judgment of physicians or other health care providers in the context of individual clinical circumstances to determine a patient's care. This algorithm should not be used to treat pregnant women. Note: Consider Clinical Trials as treatment options for eligible patients. PATHOLOGIC DIAGNOSIS INITIAL EVALUATION ESSENTIAL: 5 ● Physical exam: attention to node-bearing areas, including Waldeyer's ring, ESSENTIAL: and to size of liver and spleen ● ECOG performance status ● Hematopathology review of all slides with at least one paraffin ● B symptoms (Unexplained fever >38°C during the previous month; block or 15 unstained slides representative of the tumor. Rebiopsy if Recurrent drenching night sweats during the previous month; Weight consult material is nondiagnostic. loss >10 percent of body weight ≤ 6 months of diagnosis) ● Adequate morphology and immunophenotyping to establish 1 ● CBC with differential, LDH, BUN, creatinine, albumin, AST, diagnosis ALT, total bilirubin, alkaline phosphatase, serum calcium, uric acid ○ Paraffin Panel: CD3, CD20 and/or another pan-B-cell marker ● Beta 2 microglobulin (CD19, PAX-5, CD79a) or ● Screening for HIV 1 and 2, hepatitis B and C (HBcAb, HBsAg, HCV -

Hodgkin Lymphoma Treatment Regimens
HODGKIN LYMPHOMA TREATMENT REGIMENS (Part 1 of 5) Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced health care team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are provided only to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data become available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. Classical Hodgkin Lymphoma1 Note: All recommendations are Category 2A unless otherwise indicated. Primary Treatment Stage IA, IIA Favorable (No Bulky Disease, <3 Sites of Disease, ESR <50, and No E-lesions) REGIMEN DOSING Doxorubicin + Bleomycin + Days 1 and 15: Doxorubicin 25mg/m2 IV push + bleomycin 10units/m2 IV push + Vinblastine + Dacarbazine vinblastine 6mg/m2 IV over 5–10 minutes + dacarbazine 375mg/m2 IV over (ABVD) (Category 1)2-5 60 minutes. -

R-Eshap Patient Information Leaflet Keep All Medicine
R-ESHAP PATIENT HE-I- INFORMATION 049 LEAFLET KEEP ALL MEDICINE OUT OF THE SIGHT AND REACH OF CHILDREN R-ESHAP What is R-ESHAP? R-ESHAP is the name of a combination of cancer drugs used to treat non Hodgkin’s lymphoma (NHL). R-ESHAP is ESHAP chemotherapy with the drug rituximab. Rituximab is a type of biological therapy called a monoclonal antibody. R-ESHAP is made up of the drugs • R = Rituximab (Mabthera) • E = Etoposide - a chemotherapy drug • SH = Methylprednisolone, which is a steroid • A = Cytarabine (also known as Ara C) – a chemotherapy drug • P = Cisplatin – a chemotherapy drug How you have R-ESHAP • All the R-ESHAP drugs are clear colourless fluids. You have them into your bloodstream (intravenously). You can have them through a central line, a portacath. These are long, plastic tubes that give the drugs directly into a large vein in your chest. You have the tube put in just before your course of treatment starts and it stays in place as long as you need it. • You usually have R-ESHAP as cycles of treatment. Each cycle lasts 4 weeks. You usually have between 2 and 8 cycles. • Each cycle of treatment is given in the following way. o On the first day you have ▪ Rituximab as a slow drip (infusion) before all the other drugs – see details below ▪ Etoposide as a drip for 1 hour ▪ Methylprednisolone (steroid) as a drip for 15 to 30 minutes ▪ Cytarabine as a drip for 2 hours – this may be on the fifth day instead ▪ Cisplatin as a drip that lasts for 96 hours (4 days) o On the second, third and fourth days you ▪ Continue with your cisplatin ▪ Repeat the etoposide and methylprednisolone drips ▪ On the fifth day the cisplatin finishes and you have ▪ Another dose of methylprednisolone ▪ Another dose of cytarabine • You have no treatment for just over 3 weeks. -

(Rituxan®), Rituximab-Abbs (Truxima®), Rituximab-Pvvr (Ruxience®) Prior Authorization Drug Coverage Policy
1 Rituximab Products: Rituximab (Rituxan®), Rituximab-abbs (Truxima®), Rituximab-pvvr (Ruxience®) Prior Authorization Drug Coverage Policy Effective Date: 2/1/2021 Revision Date: n/a Review Date: 7/2/20 Lines of Business: Commercial Policy type: Prior Authorization This Drug Coverage Policy provides parameters for the coverage of rituximab (Rituxan®), rituximab-abbs (Truxima®), and rituximab-pvvr (Ruxience®). Consideration of medically necessary indications are based upon U.S. Food and Drug Administration (FDA) indications, recommended uses within the Centers of Medicare & Medicaid Services (CMS) five recognized compendia, including the National Comprehensive Cancer Network (NCCN) Drugs & Biologics Compendium (Category 1 or 2A recommendations), and peer-reviewed scientific literature eligible for coverage according to the CMS, Medicare Benefit Policy Manual, Chapter 15, section 50.4.5 titled, “Off- Label Use of Anti-Cancer Drugs and Biologics.” This policy evaluates whether the drug therapy is proven to be effective based on published evidence-based medicine. Drug Description1-3 Rituximab (Rituxan®), rituximab-abbs (Truxima®), and rituximab-pvvr (Ruxience®) are monoclonal antibodies that target the CD20 antigen expressed on the surface of pre-B and mature B- lymphocytes. Upon binding to cluster of differentiation (CD) 20, rituximab mediates B-cell lysis. Possible mechanisms of cell lysis include complement dependent cytotoxicity (CDC) and antibody dependent cell mediated cytotoxicity (ADCC). B cells are believed to play a role in the pathogenesis of rheumatoid arthritis (RA) and associated chronic synovitis. In this setting, B cells may be acting at multiple sites in the autoimmune/inflammatory process, including through production of rheumatoid factor (RF) and other autoantibodies, antigen presentation, T-cell activation, and/or proinflammatory cytokine production. -

NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines® ) Non-Hodgkin’S Lymphomas Version 2.2015
NCCN Guidelines Index NHL Table of Contents Discussion NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines® ) Non-Hodgkin’s Lymphomas Version 2.2015 NCCN.org Continue Version 2.2015, 03/03/15 © National Comprehensive Cancer Network, Inc. 2015, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN® . Peripheral T-Cell Lymphomas NCCN Guidelines Version 2.2015 NCCN Guidelines Index NHL Table of Contents Peripheral T-Cell Lymphomas Discussion DIAGNOSIS SUBTYPES ESSENTIAL: · Review of all slides with at least one paraffin block representative of the tumor should be done by a hematopathologist with expertise in the diagnosis of PTCL. Rebiopsy if consult material is nondiagnostic. · An FNA alone is not sufficient for the initial diagnosis of peripheral T-cell lymphoma. Subtypes included: · Adequate immunophenotyping to establish diagnosisa,b · Peripheral T-cell lymphoma (PTCL), NOS > IHC panel: CD20, CD3, CD10, BCL6, Ki-67, CD5, CD30, CD2, · Angioimmunoblastic T-cell lymphoma (AITL)d See Workup CD4, CD8, CD7, CD56, CD57 CD21, CD23, EBER-ISH, ALK · Anaplastic large cell lymphoma (ALCL), ALK positive (TCEL-2) or · ALCL, ALK negative > Cell surface marker analysis by flow cytometry: · Enteropathy-associated T-cell lymphoma (EATL) kappa/lambda, CD45, CD3, CD5, CD19, CD10, CD20, CD30, CD4, CD8, CD7, CD2; TCRαβ; TCRγ Subtypesnot included: · Primary cutaneous ALCL USEFUL UNDER CERTAIN CIRCUMSTANCES: · All other T-cell lymphomas · Molecular analysis to detect: antigen receptor gene rearrangements; t(2;5) and variants · Additional immunohistochemical studies to establish Extranodal NK/T-cell lymphoma, nasal type (See NKTL-1) lymphoma subtype:βγ F1, TCR-C M1, CD279/PD1, CXCL-13 · Cytogenetics to establish clonality · Assessment of HTLV-1c serology in at-risk populations. -

Drug Resistance in Non-Hodgkin Lymphomas
International Journal of Molecular Sciences Review Drug Resistance in Non-Hodgkin Lymphomas Pavel Klener 1,2,* and Magdalena Klanova 1,2 1 First Department of Internale Medicine-Hematology, University General Hospital in Prague, 128 08 Prague, Czech Republic; [email protected] 2 Institute of Pathological Physiology, First Faculty of Medicine, Charles University, Prague, 128 53 Prague, Czech Republic * Correspondence: [email protected] or [email protected] Received: 3 February 2020; Accepted: 15 March 2020; Published: 18 March 2020 Abstract: Non-Hodgkin lymphomas (NHL) are lymphoid tumors that arise by a complex process of malignant transformation of mature lymphocytes during various stages of differentiation. The WHO classification of NHL recognizes more than 90 nosological units with peculiar pathophysiology and prognosis. Since the end of the 20th century, our increasing knowledge of the molecular biology of lymphoma subtypes led to the identification of novel druggable targets and subsequent testing and clinical approval of novel anti-lymphoma agents, which translated into significant improvement of patients’ outcome. Despite immense progress, our effort to control or even eradicate malignant lymphoma clones has been frequently hampered by the development of drug resistance with ensuing unmet medical need to cope with relapsed or treatment-refractory disease. A better understanding of the molecular mechanisms that underlie inherent or acquired drug resistance might lead to the design of more effective front-line treatment algorithms based on reliable predictive markers or personalized salvage therapy, tailored to overcome resistant clones, by targeting weak spots of lymphoma cells resistant to previous line(s) of therapy. This review focuses on the history and recent advances in our understanding of molecular mechanisms of resistance to genotoxic and targeted agents used in clinical practice for the therapy of NHL. -

Approved Amendment 2 07 May 2019 Confidentiality Notice This Document Contains Confidential Information of Amgen Inc
Product: Blinatumomab Protocol Number: 20150292 Date: 07 May 2019 Page 1 of 115 Title: A Phase 2/3 Multi-center Study to Evaluate the Safety and Efficacy of Blinatumomab in Subjects With Relapsed/Refractory Aggressive B-Cell Non Hodgkin Lymphoma Amgen Protocol Number (Product Name: Blinatumomab) 20150292 EudraCT number 2016‐002044‐16 Clinical Study Sponsor: Amgen Inc. One Amgen Center Drive Thousand Oaks, California 91320 United States Phone: +1-805-447-1000 Fax: +1-805-480-4978 Key Sponsor Contact(s): Clinical Research Medical Director Email: Global Clinical Trial Manager Phone: Email: Date: 27 June 2016 Amendment 1 16 March 2017 Superseding Amendment 1: 27 April 2017 Approved Amendment 2 07 May 2019 Confidentiality Notice This document contains confidential information of Amgen Inc. This document must not be disclosed to anyone other than the site study staff and members of the institutional review board/independent ethics committee/institutional scientific review board or equivalent. The information in this document cannot be used for any purpose other than the evaluation or conduct of the clinical investigation without the prior written consent of Amgen Inc. If you have questions regarding how this document may be used or shared, call the Amgen Medical Information number: US sites, 1- 800-77-AMGEN, Canadian sites, 1-866-50-AMGEN; <<for all other countries, insert the local toll-free Medical Information number>> Amgen’s general number in the US (1-805-447-1000). NCT Number: NCT02910063 This NCT number has been applied to the document for purposes of posting on Clinical trials.gov CONFIDENTIAL Product: Blinatumomab Protocol Number: 20150292 Date: 07 May 2019 Page 2 of 115 Investigator’s Agreement I have read the attached protocol entitled “A Phase 2/3 Multi-center Study to Evaluate the Safety and Efficacy of Blinatumomab in Subjects with Relapsed/Refractory Aggressive B-Cell Non Hodgkin Lymphoma,” dated 07 May 2019 and agree to abide by all provisions set forth therein. -

Delivering Novel Targeted Therapies to Cancer Patients
NEXT-GENERATION RADIOIMMUNOTHERAPIES FOR NON-HODGKIN’S LYMPHOMA PATIENTS SEPTEMBER 2019 Nordic Nanovector ASA Kjelsåsveien 168 B, 0884 Oslo, Norway www.nordicnanovector.com IR contact: [email protected] Forward-looking statements This slide presentation contains certain forward-looking statements. These statements are based on management's current expectations and are subject to uncertainty and changes in circumstances, since they relate to events and depend on circumstances that will occur in the future and which, by their nature, will have an impact on Nordic Nanovector's business, financial condition and results of operations. The terms "anticipates", "assumes", "believes", "can", "could", "estimates", "expects", "forecasts", "intends", "may", "might", "plans", "should", "projects", "targets", "will", "would" or, in each case, their negative, or other variations or comparable terminology are used to identify forward looking statements. These forward-looking statements are not historic facts. There are a number of factors that could cause actual results and developments to differ materially from those expressed or implied in the forward-looking statements. Factors that could cause these differences include, but are not limited to, risks associated with implementation of Nordic Nanovector's strategy, risks and uncertainties associated with the development and/or approval of Nordic Nanovector's product candidates, ongoing and future clinical trials and expected trial results, the ability to commercialise Betalutin®, technology changes and new products in Nordic Nanovector's potential market and industry, Nordic Nanovector's freedom to operate (competitors patents) in respect of the products it develops, the ability to develop new products and enhance existing products, the impact of competition, changes in general economy and industry conditions, and legislative, regulatory and political factors. -

Unitedhealthcare Cancer Therapy Pathways Program Regimens
UnitedHealthcare Cancer Therapy Pathways Program Overview 2 Breast Cancer 3 Pancreatic Cancer 15 Melanoma 19 Colon/Rectal Cancer 23 Hepatobiliary Cancers 30 Lung Cancer, Small Cell 35 Lung Cancer, Non-Small Cell 40 Ovarian, Fallopian and Primary Peritoneal Cancer 56 Head and Neck Cancer 67 Multiple Myeloma 74 Lymphoma, Diffuse Large B-Cell 86 Lymphoma, Follicular 92 Lymphoma, Marginal Zone 97 Lymphoma, Mantle Cell 101 Change control 1 OVERVIEW Cancer Therapy Pathways Program With the rapid approval of new therapies, along with the rising cost of cancer care, pathways serve a critical role in the delivery of high-quality and high-value cancer treatments while reducing an unwarranted variation in care. The UnitedHealthcare Cancer Therapy Pathways Program aims to improve quality and value in cancer care by identifying anti-cancer regimens supported by evidence-based guidelines to help reduce total cost of care and improve outcomes. The program’s regimens are selected on the basis of clinical benefit (efficacy) and side-effect profile (toxicity). Among regimens with comparable efficacy and toxicity, additional consideration is given to the frequency of hospitalizations during therapy, duration of therapy, drug costs and total cost of care. Care decisions are between the physician and the patient The Cancer Therapy Pathways Program is not a substitute for the experience and judgment of a physician or other health care professional. Any clinician participating in the program must use independent medical judgment in the context of individual -

Pancreatic Cancer Treatment Using Na+/K+ Atpase
(19) & (11) EP 1 796 688 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/704 (2006.01) A61P 35/00 (2006.01) 18.05.2011 Bulletin 2011/20 (86) International application number: (21) Application number: 05794315.1 PCT/US2005/031331 (22) Date of filing: 02.09.2005 (87) International publication number: WO 2006/028969 (16.03.2006 Gazette 2006/11) (54) PANCREATIC CANCER TREATMENT USING NA+/K+ ATPASE INHIBITORS PANKREASKREBSBEHANDLUNG MIT NA+/K+-ATPASE-HEMMERN TRAITEMENT DU CANCER DU PANCREAS AU MOYEN D’INHIBITEURS D’APTASE NA+/K+ (84) Designated Contracting States: • SHARMA, Ajay AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Sudbury , MA 01776 (US) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI SK TR (74) Representative: Tomkinson, Alexandra et al Bailey Walsh & Co (30) Priority: 02.09.2004 US 606684 P 5 York Place Leeds LS1 2SD (GB) (43) Date of publication of application: 20.06.2007 Bulletin 2007/25 (56) References cited: WO-A-00/45165 WO-A-03/099011 (73) Proprietor: Bionaut Pharmaceuticals Inc WO-A-2005/060951 WO-A-2005/077925 Stoneham, MA 02180 (US) US-A1- 2005 182 105 (72) Inventors: • KHODADOUST, Mehran - c/o BT Intern. Ltd. London EC4M 7SB (GB) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

ESHAP and R-ESHAP
Lymphoma group ESHAP and R-ESHAP INDICATION Chemosensitive relapsed / refractory Hodgkin and non-Hodgkin lymphoma. Omit rituximab if CD20-negative. TREATMENT INTENT Disease Modification. PRE-ASSESSMENT 1. Ensure histology is confirmed prior to administration of chemotherapy and document in notes. 2. Record stage of disease - contrast enhanced CT scan (neck, chest, abdomen and pelvis), and/or PET-CT scan, presence or absence of B symptoms, clinical extent of disease. Bone marrow trephine if clinically indicated in Non Hodgkins lymphoma. 3. Blood tests - FBC, ESR, U&Es, LDH, urate, calcium, magnesium, creatinine, LFTs, glucose, hepatitis B core antibody and hepatitis B surface Ag, hepatitis C antibody 4. Send a "group and save" sample to transfusion and inform patient and transfusion laboratory that they will require irradiated blood products for 7 days before harvest. See ‘Guidelines for the use of blood components in adult haematology' for details. Ensure irradiation card is attached to the patient's notes and copy given to the patient. 5. Urine pregnancy test • before cycle 1 of each new chemotherapy course for women of child- bearing age unless they are post-menopausal, have been sterilised or undergone a hysterectomy. 6. ECG +/- Echo - if clinically indicated. 7. Record performance status, height and weight. 8. Consent - ensure patient has received adequate verbal and written information regarding their disease, treatment and potential side effects. Document in medical notes all information that has been given. Obtain written consent prior to treatment. 9. Fertility - it is very important the patient understands the potential risk of reduced fertility. All patients should be offered fertility advice by referring to the Oxford Fertility Unit). -
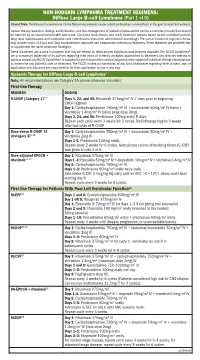
Diffuse Large B-Cell Lymphoma (Part 1 of 5)
NON-HODGKIN LYMPHOMA TREATMENT REGIMENS: Diffuse Large B-cell Lymphoma (Part 1 of 5) Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced health care team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are provided only to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data become available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. Systemic Therapy for Diffuse Large B-cell Lymphoma1 Note: All recommendations are Category 2A unless otherwise indicated. First-line Therapy REGIMEN DOSING R-CHOP (Category 1)2–7 Days 1, 22, and 43: Rituximab 375mg/m2 IV 7 days prior to beginning CHOP regimen Day 1: Cyclophosphamide 750mg/m2 IV + doxorubicin 50mg/m2 IV bolus + vincristine 1.4mg/m2 IV bolus (max dose 2mg) Days 3, 24, and 45: Prednisone 100mg orally 5 days.