Full-Text (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pdf | 90.92 Kb
PDMA PROVINCIAL DISASTER MANAGEMENT AUTHORITY Provincial Emergency Operation Center Plot 46 A, Sector B2, Phase V, Hayatabad,Peshawar Phone: (091) 9219635, 9219636, Fax: (091) 9219637 www.pdma.gov.pk No. PDMA/PEOC/DSR/2021/JulE1925 Date: 19/07/2021 KHYBER PAKHTUNKHWA DAILY SITUATION REPORT INFRA/ HUMAN INCIDENTS NATURE OF CAUSE OF CATTLE DISTRICT HUMAN LOSSES/ INJURIES INFRASTRUCTURE DAMAGES INCIDENT INCIDENT PERISHED DEATH INJURED HOUSES SCHOOLS OTHERS Mae Female Child Total Male Female Child Total Fully Partially Total Fully Partially Total Fully Partially Total Lower Dir Heavy Rain Other Damages 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 2 2 Upper Dir Heavy Rain Room Collapse 0 0 0 0 0 0 0 0 1 0 1 1 0 0 0 0 0 0 Kohat Heavy Rain Flash Flood 0 0 2 2 0 0 4 4 0 0 0 0 0 0 0 0 0 0 Total(s) 0 0 2 2 0 0 4 4 1 0 1 1 0 0 0 0 2 2 INCIDENTS DETAIL DISTRICT DETAIL OF INCIDENT RESPONSE SOURCE On 19072021 in Mouza Chorlaki, Shakardara, 07 children swept away in Flash flood due to which the following casualties took place. Death 1. Ijaz S/o Shah Hussan 2. Ruqaya d/o Shah Hussan Injured Kohat Deputy Commissioner Office Kohat 1. Tauseef Alam s/o Sajid Hussain 2. Rabia Bibi d/o Shah Hussain 3. Fahad s/o Tahir Hussain 4. Rehan s/o Tahir Hussain Missing 1. Saad s/o Tahir Hussain 02 crops i.e. -

Download Download
Int. J. Econ. Environ. Geol. Vol. Khan11 (1 )et 0 al.1- 0/Int.J.Econ.Environ.Geol.Vol.9, 2020 11(1) 01-09, 2020 Open Access Journal home page: www.econ-environ-geol.org ISSN: 2223-957X c Reservoir Potential Evaluation of the Middle Paleocene Lockhart Limestone of the Kohat Basin, Pakistan: Petrophysical Analyses Nasar Khan1, Imran Ahmad1, Muhammad Ishaq2, Irfan U. Jan3, Wasim Khan1, Muhammad Awais2, Mohsin Salam1, Bilal Khan1 1Department of Geology, University of Malakand, Pakistan 2Department of Geology, University of Swabi, Pakistan 3National Centre of Excellence in Geology, University of Peshawar, Pakistan *Email: [email protected] Received: 25 September, 2019 Accepted: 12 February, 2020 Abstract: The Lockhart Limestone is evaluated for its reservoir potential by utilizing wireline logs of Shakardara-01 well from Kohat Basin, Pakistan. The analyses showed 28.03% average volume of shale (Vsh), 25.57% average neutron porosity (NPHI), 3.31% average effective porosity (PHIE), 76% average water saturation (Sw), and 24.10% average hydrocarbon saturation (Sh) of the Lockhart Limestone in Shakardara-01 well. Based on variation in petrophysical character, the reservoir units of the Lockhart Limestone are divided into three zones i.e., zone-1, zone-2 and zone-3. Out of these zones, zone-1 and zone-2 possess a poor reservoir potential for hydrocarbons as reflected by very low effective porosity (1.40 and 2.02% respectively) and hydrocarbon saturation (15 and 5.20%), while zone-3 has a moderate reservoir potential due to its moderate effective porosity (6.50%) and hydrocarbon saturation (52%) respectively. Overall, the average effective porosity of 3.31% and hydrocarbon saturation of 24.10% as well as 28.03% volume of shale indicated poor reservoir potential of the Lockhart Limestone. -

KT15D00002-Repair Approach Street Dispensary Mirahmad Khel 220,000 220,000 220,000 220,000
District Project Description BE 2017-18 Final Budget Releases Expenditure KOHAT KT15D00002-Repair approach street dispensary MirAhmad khel 220,000 220,000 220,000 220,000 KOHAT KT15D00003-Const of Toilets, waiting shed andother 740,000 740,000 740,000 740,000 repair/maintenance of BHU Sherkot KOHAT KT15D00004-Pipe Line at BHU LandiKachai, Repair ofroof of RHC 649,000 649,000 649,000 649,000 UstarZai, Repair Work at RHC Usterzai. KOHAT KT15D00005-Maj; repair, w/s and elect, BHU KhaddarKhel 423,892 423,892 423,892 423,892 KOHAT KT15D00006-R&(M) works, const of waiting shed andG/Lat 04 No. 740,000 740,000 740,000 740,000 at RHC Lachi Bala KOHAT KT15D00007-R&(M) at BHU Ali Kach 740,000 740,000 740,000 740,000 KOHAT KT15D00008-Elect, W/S, Sanitary and building repairBHU Dolli 596,291 596,291 596,291 596,291 Banda KOHAT KT15D00009-Prov; of health related equipments andhygienic kits in 740,000 740,000 740,000 - civil hospital Shakardara Urban. Dev; works at civilHospital Shakardara. KOHAT KT15D00010-Solarization at BHU Gabari in U/CShakardara Rural-II. 740,000 740,000 740,000 - KOHAT KT15D00011-Major Repair of buildings and w/s of BHUGabari 740,000 740,000 740,000 740,000 KOHAT KT15D00012-Const;/repair works and S/F of Hand Pumpat BHU 488,122 488,122 488,122 488,122 Surgul KOHAT KT15D00013-Pavement of Dispensary street at U/CUrban III 507,090 507,090 507,090 507,000 KOHAT KT15D00014-Purchase of Hygienic kits,Purchase ofHematology 3,981,535 3,981,535 3,981,535 1,204,000 analyzer, for LMH Kohat, Purchase of Spray Pumps andInsecticides KOHAT KT15D00015-Const: -

Union Council Level
Southern K.P. Districts- Union Council Level Sher Kot Urban-4 Chorlaki Usterzai Bahadar Kot 2 Muhammad Zai Urban-3 Urban-1 Nusrat Khel Urban-2 Urban-5 Kech Banda Raisan Togh Bala Urban-6 Khan Bari Darband Ganjiano Shah Pur Kalli Bahadar Kot 1 Billitang Gumbat Sur Gul Naryab Kharmatu Khushal Garh Kahi Kotki Jarma Tora Warai Hangu Togh Serai Darsamand Dhoda Doaba HA N G U Kohat Lachi Rural Mandoori Thall Rural Muhammad Khawja Lachi Urban KO H A T Sudal Thall Urban Karbogha Dallan Gurguri Teri Jatta Ismail Khil Banda Daud Shah Empty Shakardara Urban Shakardara Nari Panos Bahadur Khel Sabir Abad Mittha Khel Karak Karak Rehmat Abad KA R A K Esak Chuntra Latambar Ghundai Mir.k.khel Chukara Dabli Aral Hathi Khel Warana Lalozai Sikandar Dand Shaho Khel Bala Kha Amandi Umar Khan 1 Asperka Wazir Ahmad Abad Chagarmash Kheli Mu Nizam Darma Khel Baloch Amandi Umar Shahbaz Azmat Khel 1 Khan 2 Hinjal Takhat Nasrati Khawaja Karab Kalli Baka Khel Muhammad Khan WazirMad Mandan Shahbaz Azmat Khel 2 Thatti Nasrati Mitta Killa Khel Masti Khel Khel Jhando Khel Jehangiri Mandew Mira Khel B A NN U Mandan Shaamshi Khel Nurar Bharat Zargar Mama Khel Azim Killa Bannu Khojary Bannu Ghoriwala Lewan Dardariz Sharawah Kaki Landidak Nar Jafar Khan Mama Khel. Jani Khel Lalozai Sikandar Khel Bala Kha Mash Masti Khani Dand Shaho Asperka Wazir Tikhtee Khel. Marmandi Azeem. Amandi Umar Khan 1 Ghundi Khan Khel. Chagarmash Kheli Mu Kot Kashmir Nizam Darma Khel Baloch Landiwah Kachi Kamar Muhammad Khan Wazir Shahbaz Azmat Khel 1 Baist Khel Amandi Umar Khan 2 Bakhmal Ahmaed Zai LA K K I Hinjal Dharka Soliman Khel Shahbaz Azmat Khel 2 MA R W A T Khawaja Mad Mandan Jhando Khel Tajazai Baka Khel Mitta Khel Killa Khel Masti Khel Pahar Khel Thal Behram Khel Mira Khel Kharu Khel Pacca Lakki Marwat Bego Khel. -

R Functional 2 332101 BHU Barh Kohat
DISTRICT KOHAT BASIC HEALTH UNIT S.No ID No Institute Name Tehsil Class Beds Locality Status 1 332099 BHU Ali Kach Lachi 1 - R Functional 2 332101 BHU Barh Kohat 1 - R Functional 3 332100 BHU Bazid Khel Kohat 1 - R Functional 4 332023 BHU Dhoda Kohat 1 - R Functional 5 332024 BHU Gabari Lachi 1 - R Functional 6 332026 BHU Kachai Kohat 1 - R Functional 7 332027 BHU Khaddar Khel Lachi 1 - R Functional 8 332028 BHU Marai Payan Kohat 1 - R Functional 9 332029 BHU Mohammad Zai Kohat 1 - R Functional 10 332030 BHU Nakband Kohat 1 - R Functional 11 332031 BHU Pershai Kohat 1 - R Functional 12 332033 BHU Shawaki Lachi 1 - R Functional 13 332035 BHU Suleman Talab Kohat 1 - R Functional 14 332036 BHU Sumari Payan Lachi 1 - R Functional 15 332037 BHU Togh Bala Kohat 1 - R Functional 16 332038 BHU Toolang Kohat 1 - R Functional 17 332039 BHU Ziarat Shaikh Aladad Kohat 1 - R Functional 18 332041 BHU Duli Banda Lachi 1 - R Functional 19 332042 BHU Kamal Khel Kohat 1 - R Functional 20 332045 BHU Sher Kot Kohat 1 - R Functional DISPENSARY 1 332050 Civil Dispy. Bezadi chikar kot Kohat 1 - U Functional 2 332052 Civil Dispy. Dartappi Lachi 1 - R Functional 3 332053 Civil DispY: Garhi mawaz khan Kohat 1 - R Functional 4 332054 Civil Dispy: Jangle khel kohat Kohat 1 - U Functional 5 332055 Civil Dispy: Kharmatoo Kohat 1 - R Functional 6 332056 Civil Dispy: Shadi khel Kohat 1 - R Functional 7 332057 Civil Dispy: Shadi pur Kohat 1 - R Functional 8 332064 Urban Dispy: Mir ahmad khel Kohat 1 - U Functional 9 332065 Urban Dispy: Lachi Lachi 1 - U Functional 10 -

Gypsum Mining & Processing District Kohat, Khyber
Cluster Study –Gypsum Mining & Processing- Kohat, Khyber Pakhtunkhwa Final Report GYPSUM MINING & PROCESSING DISTRICT KOHAT, KHYBER PAKHTUNKHWA Cluster Study Mineral Transformation Plan Vision 2025 Ministry of Planning, Development & Reforms, Government of Pakistan. Cluster Based Mineral Transformation Plan V2025- Feasibility Study Page | 1 Cluster Study –Gypsum Mining & Processing- Kohat, Khyber Pakhtunkhwa Contents 1. ABOUT THE STUDY.................................................................................................................................. 7 2. DESCRIPTION OF THE CLUSTER ......................................................................................................... 8 2.1. Introduction ....................................................................................................................................... 8 2.1.1. Strategic Location of the Cluster ........................................................................................ 8 2.2. Situational Analysis ....................................................................................................................... 10 2.2.1. Enterprise Base...................................................................................................................... 10 2.2.2. Products ................................................................................................................................... 16 2.2.3. Production Statistics ........................................................................................................... -

1 Annexure - D Names of Village / Neighbourhood Councils Alongwith Seats Detail of Khyber Pakhtunkhwa
1 Annexure - D Names of Village / Neighbourhood Councils alongwith seats detail of Khyber Pakhtunkhwa No. of General Seats in No. of Seats in VC/NC (Categories) Names of S. Names of Tehsil Councils No falling in each Neighbourhood Village N/Hood Total Col Peasants/Work S. No. Village Councils (VC) S. No. Women Youth Minority . district Council Councils (NC) Councils Councils 7+8 ers 1 2 3 4 5 6 7 8 9 10 11 12 13 Abbottabad District Council 1 1 Dalola-I 1 Malik Pura Urban-I 7 7 14 4 2 2 2 2 Dalola-II 2 Malik Pura Urban-II 7 7 14 4 2 2 2 3 Dabban-I 3 Malik Pura Urban-III 5 8 13 4 2 2 2 4 Dabban-II 4 Central Urban-I 7 7 14 4 2 2 2 5 Boi-I 5 Central Urban-II 7 7 14 4 2 2 2 6 Boi-II 6 Central Urban-III 7 7 14 4 2 2 2 7 Sambli Dheri 7 Khola Kehal 7 7 14 4 2 2 2 8 Bandi Pahar 8 Upper Kehal 5 7 12 4 2 2 2 9 Upper Kukmang 9 Kehal 5 8 13 4 2 2 2 10 Central Kukmang 10 Nawa Sher Urban 5 10 15 4 2 2 2 11 Kukmang 11 Nawansher Dhodial 6 10 16 4 2 2 2 12 Pattan Khurd 5 5 2 1 1 1 13 Nambal-I 5 5 2 1 1 1 14 Nambal-II 6 6 2 1 1 1 Abbottabad 15 Majuhan-I 7 7 2 1 1 1 16 Majuhan-II 6 6 2 1 1 1 17 Pattan Kalan-I 5 5 2 1 1 1 18 Pattan Kalan-II 6 6 2 1 1 1 19 Pattan Kalan-III 6 6 2 1 1 1 20 Sialkot 6 6 2 1 1 1 21 Bandi Chamiali 6 6 2 1 1 1 22 Bakot-I 7 7 2 1 1 1 23 Bakot-II 6 6 2 1 1 1 24 Bakot-III 6 6 2 1 1 1 25 Moolia-I 6 6 2 1 1 1 26 Moolia-II 6 6 2 1 1 1 1 Abbottabad No. -

Petrophysical Evaluation of Chanda Well-02 in Shakardara Block, Kohat, Upper Indus Basin, Pakistan
Petrophysical evaluation of Chanda well-02 in Shakardara block, Kohat, upper Indus basin, Pakistan A thesis submitted to Bahria University, Islamabad in partial fulfillment of the requirement for the degree of Bachelors of Science in Geology Asif Sultan Arslan Qaiser Seraj Alam Department of Earth and Environmental Sciences Bahria University, Islamabad 2013 DEDICATION This thesis report is dedicated to our parents and future geoscientists studying in Bahria University, Islamabad. ACKNOWLEDGEMENT We are thankful to ALLAH almighty for giving us the strength and desire to complete our thesis. We would like to express our special appreciation and thanks to our supervisor Mr. Mustafa Yar, you have been a tremendous mentor for us. We are also thankful to our Head of Department Dr. Muhammad Zafar for his kind attention and guidance and all of our teachers., who have been our kind teachers throughout our degree program especially Dr. Tahseen-ullah Khan Bangash, Mr. HammadGhani, Mr.RaieesAmjad, Mr. Anwar Qadir,Mr.AtifBukhari, Mr. Ishtiaq Noor, Mrs. Irum Khan and Ms.RifkaMunir. A special thanks to our families and friend. Words cannot express how grateful we are to our parents, for all the sacrifices that they have made. Your prayers for us were what sustained us thus far. We would also like to thank all of our friends especially Badar Habib,Yousaf bin Zahid, Umair Rashid and Usman Khan who supported us and incented us to strive towards our goals. ii ABSTRACT The study area (Chanda-02 well) is located at Latitude of 33° 13’ 55.316 ’’N and Longitude of 71° 30’ 9.419 ’’E in Shakardara area in District Kohat of the Khyber Pukhtonkhawa province. -

Te/Mof/Elect/04/2021
1 MELA OIL FIELD OIL & GAS DEVELOPMENT COMPANY LIMITED SHAKARDARA, KOHAT, KHYBER PUKTOONKHAWA PHONE # 0922-553307 FAX # 0922-553308 WEB TENDER NOTICE TE/MOF/ELECT./04/2021 1. Oil & Gas Development Company Limited (OGDCL), the largest Exploration and Production listed Company of Pakistan having operations in all the four provinces, intends to hire a 250kW Gas Generator along with standby gas generator of same capacity for Mela Oil Field. The details about the company can be obtained from website http://www.ogdcl.com. a- Sealed bids are invited under Competitive Bidding Procedure on Single Stage Two Envelope Bidding Procedure. The bid shall contain financial proposal and technical proposal in separate envelopes with clearly mentioned the caption on each envelope. b- Proposals will be evaluated as per TOR/SOR .The proposals which does not conform to the standards and criteria in TOR/SOR will be rejected or declared non responsive during technical evaluation. Financial proposals of only technically qualified suppliers will be considered and evaluated for the lowest quote. Detailed terms & conditions/schedule of requirement are mentioned in the tender documents (Annexure-A). 2. In case the Bidder fails to abide by the terms of Bid document/Bid or violates the same, it may entail temporary debarment/ black listing for three (03) years. 3. Any Bidder remained failed to full fill their commitment with OGDCL in past due to any reason or by using unfair means shall not be considered for competition. Bids Bids Opening Sr.# Tender Enquiry No. Description Submission Date & Time Date & Time Hiring of 250 kW, 415 volts, 50Hz Gas Generator 01 Prime 05 May, 2021 05 May, 2021 and 01 Standby of same 1 TE/MOF/ELECT./04/2021 capacity & fuel along with synchronization/changeover panel for Mela Oil Field on Time: 1430 hrs. -

Up-Gradation of Civil Hospital Shakardara to Category-D Hospital District Kohat (Sdg) Adp No
OFFICE OF CHIEF ENGINEER (CENTRE) COMMUNICATION & WORKS DEPARTMENT KHYBER PAKHTUNKHWA PESHAWAR 1-Police Road Peshawar Email address [email protected] 091-9211225 FAX 091-9223437 No.CEC/GST/7-3/ 41A9 Datede,e4 / 204 To The Executive Engineer, C&W Division Kohat. Subject: UP-GRADATION OF CIVIL HOSPITAL SHAKARDARA TO CATEGORY-D HOSPITAL DISTRICT KOHAT (SDG) ADP NO. 537/160233 (2017-18).. Reference: S.E. C&W Circle Kohat letter No. 2089/15, dated 06-12-2017. As recommended by Procurement Committee, the discounted bid costing Rs. 197,655,664.35 (Rupees One Hundred & Ninety Seven Million, Six Hundred, Fifty Five Thousand, Six Hundred & Sixty Four point Three Five) based on the lowest rate viz 01% Below (One percent below on BOQ cost based on MRS-2017 & on NSI) offered by M/S Younas & Brothers (Waqas JV Younas), Government Contractor for the subject cited work is hereby approved on the basis of documents submitted by you and forwarded by Superintending Engineer C&W Circle Kohat, subject to the following conditions:- 1. Fulfillment of all codal & financial formalities. 2. The Executive Engineer/Sub Divisional Officer incharge are responsible for the execution of the work according to the specifications, scope of work & quantities of all items as provided in administratively approved PC-I/ Cost estimate. 3. The work may be completed in stipulated period and technical sanction from the competent authority must be obtained before commencement of the work. The work order shall only be issued after fulfillment of all codal formalities. 4. The contractor may be directed to sign the contract agreement and the work shall commence after availability of funds. -

Curriculum Vitae
AUDIT REPORT ON THE ACCOUNTS OF DISTRICT GOVERNMENT KOHAT AUDIT YEAR 2013-14 AUDITOR GENERAL OF PAKISTAN TABLE OF CONTENTS ABBREVIATIONS AND ACRONYMS .........................................................................................i Preface ............................................................................................................................................. ii EXECUTIVE SUMMARY ............................................................................................................ iii SUMMARY TABLES & CHARTS ............................................................................................. vii Table 1: Audit Work Statistics ................................................................................................. vii Table 2: Audit observations Classified by Categories .............................................................. vii Table 3: Outcome Statistics ..................................................................................................... viii Table 4: Table of Irregularities pointed out ................................................................................ix CHAPTER 1 .................................................................................................................................... 1 1.1 District Government Kohat ........................................................................................ 1 1.1.1 Introduction .............................................................................................................. -
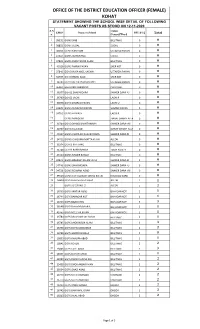
KOHAT STATEMENT SHOWING the SCHOOL WISE DETAIL of FOLLOWING VACANT POSTS AS STOOD on 12-11-2020 S.N Union EMIS Name of School PST B-12 O
OFFICE OF THE DISTRICT EDUCATION OFFICER (FEMALE) KOHAT STATEMENT SHOWING THE SCHOOL WISE DETAIL OF FOLLOWING VACANT POSTS AS STOOD ON 12-11-2020 S.N Union EMIS Name of School PST B-12 o. Council/Ward Total 1 38355 GGMS SIAB BILLITANG 0 0 2 38832 GGMS SUDAL SUDAL 0 0 3 38360 GGMS KAM SAM USTARZAI PAYAN 0 0 4 41641 GGMS WARSHAND SUDAL 0 0 5 37841 GGMS DHOK NOOR ALAM BILLITANG 0 0 6 40116 GGMS MARAI PAYAN SHER KOT 0 0 7 37840 GGHS MUSA KHEL KACHAI USTARZAI PAYAN 0 0 8 34739 GGHS MARAI BALA SHER KOT 0 0 9 34743 GGHS KACHAI HASSAN KHEL USTARZAI PAYAN 0 0 10 36381 GGHS KERI SHEIKHAN CHORLAKI 0 0 11 36377 GGHSS SHAKARDARA SHAKER DARA -U 0 0 12 36709 GGHSS LACHI LACHI R 0 0 13 36948 GGHS GHURZAI PAYAN LACHI U 0 0 14 41635 GGMS SUMMARI PAYAN SUMARI PAYAN 0 0 15 34761 GGMS ALI KACH LACHI R 0 0 16 GGMS MANDONI ZIARAT SHAIKH ALLAH DAD0 0 17 34740 GGHS DHAND BAKHTAWARA SHAKER DARA- RII 0 0 18 34757 GGHS Z.S.A.DAD ZIARAT SHAIKH ALLAH DAD0 0 19 40117 GGMS CHORLAKI SHAKER DARA SHAKER DARA-RI 0 0 20 34752 GGMS CHASHMA MATTA KHAN ALI ZAI 0 0 21 36374 GGHSS BILLITANG BILLITANG 0 0 22 36708 GGHSS BABRI BANDA TOGH BALA -II 0 0 23 41690 GGMS RAZGIR BANDA BILLITANG 0 0 24 39521 GGMS BRAGHZI KALAN LACHI SHAKER DARA-RI 0 0 25 34746 GGMS SHAKARDARA SHAKER DARA -U 0 0 26 34758 GGMS REHMAN ABAD SHAKER DARA- RII 0 0 27 39513 GGMS GUL HASSAN BANDA KOHAT KHUSHAL GARH 0 0 28 16824 GGPS Kamsam No.1Kohat ALI ZAI 1 1 29 GGPS ALI ZAI NO.