Biogeography and Taxonomy of Pyrenomycetous Fungi 3. the Area Around the Sea of Japan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Insight Into the Phylogeographic Pattern Of
New insight into the phylogeographic pattern of Liriodendron chinense (Magnoliaceae) revealed by chloroplast DNA: east–west lineage split and genetic mixture within western subtropical China Aihong Yang, Yongda Zhong, Shujuan Liu, Lipan Liu, Tengyun Liu, Yanqiang Li and Faxin Yu The Key Laboratory of Horticultural Plant Genetic and Improvement of Jiangxi, Institute of Biological Resources, Jiangxi Academy of Sciences, Nanchang, Jiangxi, China ABSTRACT Background: Subtropical China is a global center of biodiversity and one of the most important refugia worldwide. Mountains play an important role in conserving the genetic resources of species. Liriodendron chinense is a Tertiary relict tree largely endemic to subtropical China. In this study, we aimed to achieve a better understanding of the phylogeographical pattern of L. chinense andtoexploretheroleofmountainsintheconservationofL. chinense genetic resources. Methods: Three chloroplast regions (psbJ-petA, rpl32-ndhF, and trnK5’-matK) were sequenced in 40 populations of L. chinense for phylogeographical analyses. Relationships among chloroplast DNA (cpDNA) haplotypes were determined using median-joining networks, and genetic structure was examined by spatial analysis of molecular variance (SAMOVA). The ancestral area of the species was reconstructed using the Bayesian binary Markov Chain Monte Carlo (BBM) method according to its geographic distribution and a maximum parsimony (MP) tree based on Bayesian methods. Results: Obvious phylogeographic structure was found in L. chinense. SAMOVA Submitted 13 September 2018 revealed seven groups matching the major landscape features of the L. chinense Accepted 26 December 2018 Published 1 February 2019 distribution area. The haplotype network showed three clades distributed in the eastern, southwestern, and northwestern regions. Separate northern and southern Corresponding author Faxin Yu, [email protected] refugia were found in the Wu Mountains and Yungui Plateau, with genetic admixture in the Dalou Mountains and Wuling Mountains. -

Potential Antioxidant and Antiproliferative Activities of a Hot-Water Extract from the Root of Tonh Khidum
ONCOLOGY LETTERS 1: 383-387, 2010 383 Potential antioxidant and antiproliferative activities of a hot-water extract from the root of Tonh khidum JIQIANG LIU, ZHENYA ZHANG, GUOQING XING, HONGHAI HU, NORIO SUGIURA and INTABON KEO Graduate School of Life and Environmental Sciences, University of Tsukuba, Tsukuba 305-8572, Japan Received September 29, 2009; Accepted December 4, 2009 DOI: 10.3892/ol_00000068 Abstract. In this study, for the first time, the possible antioxidant constituents and bioactivities. In particular, no investigation and antiproliferative activities of a hot-water extract (TW100) has been reported in the literature regarding the antioxidant from the root of Tonh khidum (Actinidia kolomikta Maxim) and antiproliferative properties of Tonh khidum in vitro. were examined in vitro. Total phenolic compound, 1,1-diphenyl- It is commonly accepted that under situations of oxidative 2-picrylhydrazyl (DPPH) radical-scavenging activity and stress, reactive oxygen species (ROS) such as superoxide ● - ● ● ● superoxide dismutase (SOD)-like activity assays were utilized to ( O2 , OOH), hydroxyl ( OH) and peroxyl (ROO ) radicals investigate its antioxidant activity. As a result, TW100 showed a are generated. ROS play an important role in degenerative strong antioxidant activity. The total phenolic content of TW100 or pathological processes such as aging (1), cancer, coronary was 143 µg gallic acid equivalents/mg. The SOD-like activity heart disease, Alzheimer's disease (2-4), neurodegenerative of TW100 was 666,667 U/g extract, and the DPPH radical- disorders, atherosclerosis, cataracts and inflammation (5). scavenging activity was 129 µg/ml at EC50 which was one Generally, cells possess endogenous systems [superoxide third of vitamin E (40 µg/ml). -

Bio 308-Course Guide
COURSE GUIDE BIO 308 BIOGEOGRAPHY Course Team Dr. Kelechi L. Njoku (Course Developer/Writer) Professor A. Adebanjo (Programme Leader)- NOUN Abiodun E. Adams (Course Coordinator)-NOUN NATIONAL OPEN UNIVERSITY OF NIGERIA BIO 308 COURSE GUIDE National Open University of Nigeria Headquarters 14/16 Ahmadu Bello Way Victoria Island Lagos Abuja Office No. 5 Dar es Salaam Street Off Aminu Kano Crescent Wuse II, Abuja e-mail: [email protected] URL: www.nou.edu.ng Published by National Open University of Nigeria Printed 2013 ISBN: 978-058-434-X All Rights Reserved Printed by: ii BIO 308 COURSE GUIDE CONTENTS PAGE Introduction ……………………………………......................... iv What you will Learn from this Course …………………............ iv Course Aims ……………………………………………............ iv Course Objectives …………………………………………....... iv Working through this Course …………………………….......... v Course Materials ………………………………………….......... v Study Units ………………………………………………......... v Textbooks and References ………………………………........... vi Assessment ……………………………………………….......... vi End of Course Examination and Grading..................................... vi Course Marking Scheme................................................................ vii Presentation Schedule.................................................................... vii Tutor-Marked Assignment ……………………………….......... vii Tutors and Tutorials....................................................................... viii iii BIO 308 COURSE GUIDE INTRODUCTION BIO 308: Biogeography is a one-semester, 2 credit- hour course in Biology. It is a 300 level, second semester undergraduate course offered to students admitted in the School of Science and Technology, School of Education who are offering Biology or related programmes. The course guide tells you briefly what the course is all about, what course materials you will be using and how you can work your way through these materials. It gives you some guidance on your Tutor- Marked Assignments. There are Self-Assessment Exercises within the body of a unit and/or at the end of each unit. -

ASCOMYCOTA) EN ARGENTINA Y NUEVOS REGISTROS PARA EL PAÍS Darwiniana, Vol
Darwiniana ISSN: 0011-6793 [email protected] Instituto de Botánica Darwinion Argentina Robles, Carolina A.; D’Jonsiles, María F.; Romano, Gonzalo M.; Hladki, Adriana; Carmarán, Cecilia C. DIVERSIDAD Y DISTRIBUCIÓN DE DIATRYPACEAE (ASCOMYCOTA) EN ARGENTINA Y NUEVOS REGISTROS PARA EL PAÍS Darwiniana, vol. 4, núm. 2, diciembre, 2016, pp. 263-276 Instituto de Botánica Darwinion Buenos Aires, Argentina Disponible en: http://www.redalyc.org/articulo.oa?id=66949983004 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto DARWINIANA, nueva serie 4(2): 263-276. 2016 Versión final, efectivamente publicada el 31 de diciembre de 2016 DOI: 10.14522/darwiniana.2016.42.687 ISSN 0011-6793 impresa - ISSN 1850-1699 en línea DIVERSIDAD Y DISTRIBUCIÓN DE DIATRYPACEAE (ASCOMYCOTA) EN ARGENTINA Y NUEVOS REGISTROS PARA EL PAÍS Carolina A. Robles1, María F. D’Jonsiles1, Gonzalo M. Romano2, Adriana Hladki3 & Cecilia C. Carmarán1 1 INMIBO UBA-CONICET, Departamento de Biodiversidad y Biología Experimental, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Ciudad Universitaria, Pabellón II, Piso 4, C1428EHA Ciudad Autónoma de Buenos Aires, Argentina. [email protected] (autor corresponsal). 2 Departamento de Biología, Facultad de Ciencias Naturales, Universidad Nacional de la Patagonia San Juan Bos- co, CONICET, Ruta 259 Km 16, 9200 Esquel, Chubut, Argentina. 3 Laboratorio de Micología, Fundación Miguel Lillo, Miguel Lillo 251, 4000 San Miguel de Tucumán, Tucumán, Argentina. -

BIOGEOGRAPHIC RECONSTRUCTION of the GENUS FERULA INFERRED from ANALYSES of Nrdna and Cpdna SEQUENCES
IRANIAN JOURNAL OF BOTANY 25 (2), 2019 DOI: 10.22092/ijb.2019.126389.1241 BIOGEOGRAPHIC RECONSTRUCTION OF THE GENUS FERULA INFERRED FROM ANALYSES OF nrDNA AND cpDNA SEQUENCES M. Panahi Received 2019. 05. 22; accepted for publication 2019. 11. 20 Panahi, M., 2019. 12. 30: Biogeographic reconstruction of the genus Ferula inferred from analyses of nrDNA and cpDNA sequences. -Iran. J. Bot. 25 (2): 79-94. Tehran. The divergence time of the largely Asian element, the genus Ferula L. (subtribe Ferulinae, tribe Scandiceae, Apiaceae) was initially analyzed using nrDNA, internal transcribed spacer (ITS) sequence data followed with three regions of cpDNA (rps16 and rpoC1 introns and rpoB-trnC intergenic spacers) from 141 representatives of subtribe Ferulinae (Ferula, Dorema, Leutea) and relatives. Further analyses of the biogeographical history of the Ferula group were carried out using BEAST, S-DIVA and BBM in RASP on all datasets. The results suggested that the initial split within Ferulinae occurred during Early Pliocene about 4.8 Ma and earlier ancestor of Ferula group has been originated mostly in Armeno-Iranian province. One descendent giving rises to the Ferula clade and another descendent subsequently underwent further divergence to account for Leutea lineages about 1.8-2 Ma. The rapid diversification within Ferulinae clade has occurred since the Middle Pliocene. Subsequent diversification of the Ferula clade intensified in the Irano-Turanian region during the late Pliocene in the Central Asian zone and toward west in Mediterranean zone during early Pleistocene. The ancestor of Chinese group of Ferula spread from Central Asia to eastern Asia in the Pliocene (2.2-4 Ma). -

Transboundary Cooperation for Nature Conservation World Trends and Ways Forward in Northeast Asia
NEASPEC WORKING PAPER Transboundary Cooperation for Nature Conservation World Trends and Ways Forward in Northeast Asia February 2015 This working paper was prepared by Alexandre Edwardes, intern for NEASPEC, under the supervision of Sangmin Nam, Deputy Head, East and North-East Asia Office of the ESCAP. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. This paper follows United Nations practice in references to countries. Where there are space constraints, some country names have been abbreviated. Transboundary Cooperation for Nature Conservation Alexandre Edwardes Transboundary Cooperation for Nature Conservation World Trends and Ways Forward in Northeast Asia (May 2015) Table of Contents 1. Introduction ........................................................................................................................................... 3 2. Transboundary Conservation Initiatives Worldwide ............................................................................. 4 a. Brief history and current trends in transboundary conservation ...................................................... 4 b. Definitions and designations of transboundary conservation initiatives .......................................... 5 • Transboundary Protected Areas .................................................................................................... -

Lichens and Associated Fungi from Glacier Bay National Park, Alaska
The Lichenologist (2020), 52,61–181 doi:10.1017/S0024282920000079 Standard Paper Lichens and associated fungi from Glacier Bay National Park, Alaska Toby Spribille1,2,3 , Alan M. Fryday4 , Sergio Pérez-Ortega5 , Måns Svensson6, Tor Tønsberg7, Stefan Ekman6 , Håkon Holien8,9, Philipp Resl10 , Kevin Schneider11, Edith Stabentheiner2, Holger Thüs12,13 , Jan Vondrák14,15 and Lewis Sharman16 1Department of Biological Sciences, CW405, University of Alberta, Edmonton, Alberta T6G 2R3, Canada; 2Department of Plant Sciences, Institute of Biology, University of Graz, NAWI Graz, Holteigasse 6, 8010 Graz, Austria; 3Division of Biological Sciences, University of Montana, 32 Campus Drive, Missoula, Montana 59812, USA; 4Herbarium, Department of Plant Biology, Michigan State University, East Lansing, Michigan 48824, USA; 5Real Jardín Botánico (CSIC), Departamento de Micología, Calle Claudio Moyano 1, E-28014 Madrid, Spain; 6Museum of Evolution, Uppsala University, Norbyvägen 16, SE-75236 Uppsala, Sweden; 7Department of Natural History, University Museum of Bergen Allégt. 41, P.O. Box 7800, N-5020 Bergen, Norway; 8Faculty of Bioscience and Aquaculture, Nord University, Box 2501, NO-7729 Steinkjer, Norway; 9NTNU University Museum, Norwegian University of Science and Technology, NO-7491 Trondheim, Norway; 10Faculty of Biology, Department I, Systematic Botany and Mycology, University of Munich (LMU), Menzinger Straße 67, 80638 München, Germany; 11Institute of Biodiversity, Animal Health and Comparative Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G12 8QQ, UK; 12Botany Department, State Museum of Natural History Stuttgart, Rosenstein 1, 70191 Stuttgart, Germany; 13Natural History Museum, Cromwell Road, London SW7 5BD, UK; 14Institute of Botany of the Czech Academy of Sciences, Zámek 1, 252 43 Průhonice, Czech Republic; 15Department of Botany, Faculty of Science, University of South Bohemia, Branišovská 1760, CZ-370 05 České Budějovice, Czech Republic and 16Glacier Bay National Park & Preserve, P.O. -

RCN #33 21/8/03 13:57 Page 1
RCN #33 21/8/03 13:57 Page 1 No. 33 Summer 2003 Special issue: The Transformation of Protected Areas in Russia A Ten-Year Review PROMOTING BIODIVERSITY CONSERVATION IN RUSSIA AND THROUGHOUT NORTHERN EURASIA RCN #33 21/8/03 13:57 Page 2 CONTENTS CONTENTS Voice from the Wild (Letter from the Editors)......................................1 Ten Years of Teaching and Learning in Bolshaya Kokshaga Zapovednik ...............................................................24 BY WAY OF AN INTRODUCTION The Formation of Regional Associations A Brief History of Modern Russian Nature Reserves..........................2 of Protected Areas........................................................................................................27 A Glossary of Russian Protected Areas...........................................................3 The Growth of Regional Nature Protection: A Case Study from the Orlovskaya Oblast ..............................................29 THE PAST TEN YEARS: Making Friends beyond Boundaries.............................................................30 TRENDS AND CASE STUDIES A Spotlight on Kerzhensky Zapovednik...................................................32 Geographic Development ........................................................................................5 Ecotourism in Protected Areas: Problems and Possibilities......34 Legal Developments in Nature Protection.................................................7 A LOOK TO THE FUTURE Financing Zapovedniks ...........................................................................................10 -

Moss Diversity Distribution Patterns and Agglomerates of Local Floras in the Russian Far East
Botanica Pacifica. A journal of plant science and conservation. 2017. 6(2): 21–33 DOI: 10.17581/bp.2017.06201 Moss diversity distribution patterns and agglomerates of local floras in the Russian Far East Olga Yu. Pisarenko 1* & Vadim A. Bakalin 2 Olga Yu. Pisarenko1* ABSTRACT email: [email protected] Published materials on the mosses of the Russian Far East are summarized. Nine Vadim A. Bakalin 2 hund red and thirty species of mosses were revealed, and a bibliography is provi email: [email protected] ded for each taxon. The distribution of each taxon within 39 spatial units (5×5 de grees latitude/longitude) is analyzed. The list for each square was regarded 1 Central Siberian Botanical Garden, SB as the flo ra of minimal size involved in analysis. Analysis of interrelationships RAS, Novosibirsk 630090 Russia be tween each minimal flora has revealed seven floristic associations that corres 2 Botanical GardenInstitute FEB RAS, pond to the following territories: Beringian Chukotka, the continental part of Vladivostok 690024 Russia Chu kotka Autonomous District and continental part of Magadan Province, nor thern coast of the Sea of Okhotsk, Kamchatka and adjacent islands, Sakhalin and southern Kurils, Russian Manchuria, and the rest part of continental sou thern Russian Far East. Centers of moss species diversity are considered. * corresponding author Keywords: Russian Far East, mosses, bryoflora, distribution patterns, diversity, con servation, phytogeography Manuscript received: 17.02.2017 РЕЗЮМЕ Review completed: 13.09.2017 Писаренко О.Ю., Бакалин В.А. Закономерности распространения Accepted for publication: 18.09.2017 раз нообразия мхов и естественные агломераты локальных моховых Published online: 19.09.2017 флор на российском Дальнем Востоке. -

Myconet Volume 14 Part One. Outine of Ascomycota – 2009 Part Two
(topsheet) Myconet Volume 14 Part One. Outine of Ascomycota – 2009 Part Two. Notes on ascomycete systematics. Nos. 4751 – 5113. Fieldiana, Botany H. Thorsten Lumbsch Dept. of Botany Field Museum 1400 S. Lake Shore Dr. Chicago, IL 60605 (312) 665-7881 fax: 312-665-7158 e-mail: [email protected] Sabine M. Huhndorf Dept. of Botany Field Museum 1400 S. Lake Shore Dr. Chicago, IL 60605 (312) 665-7855 fax: 312-665-7158 e-mail: [email protected] 1 (cover page) FIELDIANA Botany NEW SERIES NO 00 Myconet Volume 14 Part One. Outine of Ascomycota – 2009 Part Two. Notes on ascomycete systematics. Nos. 4751 – 5113 H. Thorsten Lumbsch Sabine M. Huhndorf [Date] Publication 0000 PUBLISHED BY THE FIELD MUSEUM OF NATURAL HISTORY 2 Table of Contents Abstract Part One. Outline of Ascomycota - 2009 Introduction Literature Cited Index to Ascomycota Subphylum Taphrinomycotina Class Neolectomycetes Class Pneumocystidomycetes Class Schizosaccharomycetes Class Taphrinomycetes Subphylum Saccharomycotina Class Saccharomycetes Subphylum Pezizomycotina Class Arthoniomycetes Class Dothideomycetes Subclass Dothideomycetidae Subclass Pleosporomycetidae Dothideomycetes incertae sedis: orders, families, genera Class Eurotiomycetes Subclass Chaetothyriomycetidae Subclass Eurotiomycetidae Subclass Mycocaliciomycetidae Class Geoglossomycetes Class Laboulbeniomycetes Class Lecanoromycetes Subclass Acarosporomycetidae Subclass Lecanoromycetidae Subclass Ostropomycetidae 3 Lecanoromycetes incertae sedis: orders, genera Class Leotiomycetes Leotiomycetes incertae sedis: families, genera Class Lichinomycetes Class Orbiliomycetes Class Pezizomycetes Class Sordariomycetes Subclass Hypocreomycetidae Subclass Sordariomycetidae Subclass Xylariomycetidae Sordariomycetes incertae sedis: orders, families, genera Pezizomycotina incertae sedis: orders, families Part Two. Notes on ascomycete systematics. Nos. 4751 – 5113 Introduction Literature Cited 4 Abstract Part One presents the current classification that includes all accepted genera and higher taxa above the generic level in the phylum Ascomycota. -

Molecular Systematics of the Sordariales: the Order and the Family Lasiosphaeriaceae Redefined
Mycologia, 96(2), 2004, pp. 368±387. q 2004 by The Mycological Society of America, Lawrence, KS 66044-8897 Molecular systematics of the Sordariales: the order and the family Lasiosphaeriaceae rede®ned Sabine M. Huhndorf1 other families outside the Sordariales and 22 addi- Botany Department, The Field Museum, 1400 S. Lake tional genera with differing morphologies subse- Shore Drive, Chicago, Illinois 60605-2496 quently are transferred out of the order. Two new Andrew N. Miller orders, Coniochaetales and Chaetosphaeriales, are recognized for the families Coniochaetaceae and Botany Department, The Field Museum, 1400 S. Lake Shore Drive, Chicago, Illinois 60605-2496 Chaetosphaeriaceae respectively. The Boliniaceae is University of Illinois at Chicago, Department of accepted in the Boliniales, and the Nitschkiaceae is Biological Sciences, Chicago, Illinois 60607-7060 accepted in the Coronophorales. Annulatascaceae and Cephalothecaceae are placed in Sordariomyce- Fernando A. FernaÂndez tidae inc. sed., and Batistiaceae is placed in the Euas- Botany Department, The Field Museum, 1400 S. Lake Shore Drive, Chicago, Illinois 60605-2496 comycetes inc. sed. Key words: Annulatascaceae, Batistiaceae, Bolini- aceae, Catabotrydaceae, Cephalothecaceae, Ceratos- Abstract: The Sordariales is a taxonomically diverse tomataceae, Chaetomiaceae, Coniochaetaceae, Hel- group that has contained from seven to 14 families minthosphaeriaceae, LSU nrDNA, Nitschkiaceae, in recent years. The largest family is the Lasiosphaer- Sordariaceae iaceae, which has contained between 33 and 53 gen- era, depending on the chosen classi®cation. To de- termine the af®nities and taxonomic placement of INTRODUCTION the Lasiosphaeriaceae and other families in the Sor- The Sordariales is one of the most taxonomically di- dariales, taxa representing every family in the Sor- verse groups within the Class Sordariomycetes (Phy- dariales and most of the genera in the Lasiosphaeri- lum Ascomycota, Subphylum Pezizomycotina, ®de aceae were targeted for phylogenetic analysis using Eriksson et al 2001). -
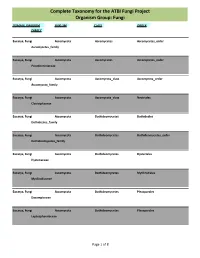
Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi
Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi DOMAIN, KINGDOM PHYLUM CLASS ORDER FAMILY Eucarya, Fungi Ascomycota Ascomycetes Ascomycetes_order Ascomycetes_family Eucarya, Fungi Ascomycota Ascomycetes Ascomycetes_order Pseudeurotiaceae Eucarya, Fungi Ascomycota Ascomycota_class Ascomycota_order Ascomycota_family Eucarya, Fungi Ascomycota Ascomycota_class Nectriales Clavicipitaceae Eucarya, Fungi Ascomycota Dothideomycetes Dothideales Dothideales_family Eucarya, Fungi Ascomycota Dothideomycetes Dothideomycetes_order Dothideomycetes_family Eucarya, Fungi Ascomycota Dothideomycetes Hysteriales Hysteriaceae Eucarya, Fungi Ascomycota Dothideomycetes Mytilinidiales Mytilinidiaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Dacampiaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Leptosphaeriaceae Page 1 of 8 Complete Taxonomy for the ATBI Fungi Project Organism Group: Fungi DOMAIN, KINGDOM PHYLUM CLASS ORDER FAMILY Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Lophiostomataceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Massarinaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Melanommataceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleomassariaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporaceae Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporales Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Pleosporales_family Eucarya, Fungi Ascomycota Dothideomycetes Pleosporales Sporormiaceae Eucarya, Fungi Ascomycota Dothideomycetes