Preparation of a Buffer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Choosing Buffers Based on Pka in Many Experiments, We Need a Buffer That Maintains the Solution at a Specific Ph. We May Need An
Choosing buffers based on pKa In many experiments, we need a buffer that maintains the solution at a specific pH. We may need an acidic or a basic buffer, depending on the experiment. The Henderson-Hasselbalch equation can help us choose a buffer that has the pH we want. pH = pKa + log([conj. base]/[conj. acid]) With equal amounts of conjugate acid and base (preferred so buffers can resist base and acid equally), then … pH = pKa + log(1) = pKa + 0 = pKa So choose conjugates with a pKa closest to our target pH. Chemistry 103 Spring 2011 Example: You need a buffer with pH of 7.80. Which conjugate acid-base pair should you use, and what is the molar ratio of its components? 2 Chemistry 103 Spring 2011 Practice: Choose the best conjugate acid-base pair for preparing a buffer with pH 5.00. What is the molar ratio of the buffer components? 3 Chemistry 103 Spring 2011 buffer capacity: the amount of strong acid or strong base that can be added to a buffer without changing its pH by more than 1 unit; essentially the number of moles of strong acid or strong base that uses up all of the buffer’s conjugate base or conjugate acid. Example: What is the capacity of the buffer solution prepared with 0.15 mol lactic acid -4 CH3CHOHCOOH (HA, Ka = 1.0 x 10 ) and 0.20 mol sodium lactate NaCH3CHOHCOO (NaA) and enough water to make 1.00 L of solution? (from previous lecture notes) 4 Chemistry 103 Spring 2011 Review of equivalence point equivalence point: moles of H+ = moles of OH- (moles of acid = moles of base, only when the acid has only one acidic proton and the base has only one hydroxide ion). -

Buffers a Guide for the Preparation and Use of Buffers in Biological Systems Calbiochem® Buffers a Guide for the Preparation and Use of Buffers in Biological Systems
Buffers A guide for the preparation and use of buffers in biological systems Calbiochem® Buffers A guide for the preparation and use of buffers in biological systems Chandra Mohan, Ph.D. EMD, San Diego, California © EMD, an affiliate of Merck KGaA, Darmstadt, Germany. All rights reserved. A word to our valued customers We are pleased to present to you the newest edition of Buffers: A Guide for the Preparation and Use of Buffers in Biological Systems. This practical resource has been especially revamped for use by researchers in the biological sciences. This publication is a part of our continuing commitment to provide useful product information and exceptional service to you, our customers. You will find this booklet a highly useful resource, whether you are just beginning your research work or training the newest researchers in your laboratory. Over the past several years, EMD Biosciences has clearly emerged as a world leader in providing highly innovative products for your research needs in Signal Transduction, including the areas of Cancer Biology, Alzheimer’s Disease, Diabetes, Hypertension, Inflammation, and Apoptosis. Please call us today for a free copy of our LATEST Catalog that includes tools for signal transduction and life science research. If you have used our products in the past, we thank you for your support and confidence in our products, and if you are just beginning your research career, please call us and give us the opportunity to demonstrate our exceptional customer and technical service. Corrine Fetherston Sr. Director, Marketing ii Table of Contents: Why does Calbiochem® Biochemicals Publish a Booklet on Buffers? . -

AMMONIA BUFFER SOLUTION MSDS CAS-No
AMMONIA BUFFER SOLUTION MSDS CAS-No.: MSDS MATERIAL SAFETY DATA SHEET (MSDS) SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Mixture : Product code : 01077 1.2. Relevant identified uses of the substance or mixture and uses advised against 1.2.1. Relevant identified uses Use of the substance/mixture : Laboratory chemicals, Manufacture of substances 1.2.2. Uses advised against No additional information available 1.3. Details of the supplier of the safety data sheet LOBA CHEMIE PVT.LTD. 107 Wode House Road, Jehangir Villa, Colaba 400005 Mumbai - INDIA T +91 22 6663 6663 - F +91 22 6663 6699 [email protected] - www.lobachemie.com 1.4. Emergency telephone number Emergency number : + 91 22 6663 6663 (9:00am - 6:00 pm) SECTION 2: Hazards identification 2.1. Classification of the substance or mixture Classification according to Regulation (EC) No. 1272/2008 [CLP]Mixtures/Substances: SDS EU 2015: According to Regulation (EU) 2015/830 (REACH Annex II) Skin corrosion/irritation, H314 Category 1B Specific target organ H335 toxicity — Single exposure, Category 3, Respiratory tract irritation Hazardous to the aquatic H400 environment — Acute Hazard, Category 1 Full text of hazard classes and H-statements : see section 16 Adverse physicochemical, human health and environmental effects No additional information available www.lobachemie.com 21/04/2016 1/9 AMMONIA BUFFER SOLUTION Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH) with its amendment Regulation (EU) 2015/830 2.2. Label elements Labelling according to Regulation (EC) No. 1272/2008 [CLP] Extra labelling to displayExtra classification(s) to display Hazard pictograms (CLP) : GHS05 GHS07 GHS09 Signal word (CLP) : Danger Hazard statements (CLP) : H314 - Causes severe skin burns and eye damage. -

Solvent Effects on the Thermodynamic Functions of Dissociation of Anilines and Phenols
University of Wollongong Research Online University of Wollongong Thesis Collection 1954-2016 University of Wollongong Thesis Collections 1982 Solvent effects on the thermodynamic functions of dissociation of anilines and phenols Barkat A. Khawaja University of Wollongong Follow this and additional works at: https://ro.uow.edu.au/theses University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Recommended Citation Khawaja, Barkat A., Solvent effects on the thermodynamic functions of dissociation of anilines and phenols, Master of Science thesis, Department of Chemistry, University of Wollongong, 1982. -

Buffers and Buffer Capacity
BUFFERS AND BUFFER CAPACITY By Dr. Jan Pláteník Principle of buffering A buffer solution is a solution that resists changes in pH either when diluted or when limited amounts of acid or base are added to it. Such a solution can be prepared by combining a weak acid and its salt with a strong base (conjugated base) or, analogously, a weak base and its salt with a strong acid (conjugated acid). For example: Acetate buffer : CH 3COOH (the weak acid) + CH 3COONa (the salt, conjugated base) Phosphate buffer : NaH 2PO 4 (the weak acid) + Na 2HPO 4 (the salt, conjugated base) Tris buffer: (Tris: Tris [2-amino-2-(hydroxymethyl)-propan-1,3-diol)] , an organic base) The Henderson-Hasselbalch equation describes the behavior of such a buffer and for the mixture of a weak acid and its salt with a strong base (conjugated base) it has the form: cs pH = pK a + log cac pK a negative logarithm of the dissociation constant for the weak acid, cs substance concentration of the salt (conjugated base), cac substance concentration of the weak acid (conjugated acid). Graphically the Henderson-Hasselbalch equation plotted as the acid : conjugated base ratio vs. pH of buffer actually constitutes the titration curve of the weak acid (see figure on the next page). Note also that for the acid : base ratio 1:1 the pH of buffer just equals the pK a (this is valid for uni-univalent systems, e.g., CH 3COOH/CH 3COONa, NaH 2PO 4/Na 2HPO 4). 1 Titration curve of sodium phosphate buffer 10 9 8 pH = pKa = 7.21 7 pHof buffer 6 5 9:1 8:2 7:3 6:4 1:1 4:6 3:7 2:8 1:9 Acid : base ratio The equation for a weak base and its salt with a strong acid (conjugated acid) has the form: cb pH = pK w − pK b + log cs pK b negative logarithm of the dissociation constant for the weak base, cb substance concentration of the base, cs substance concentration of the salt (conjugated acid), -14 pK w = 14 = − log 10 (ionic product of water). -

CHAPTER 17: Advanced Acid-Base Equilibria
Chapter 17 Advanced Acid-Base Equilibria SY 4/12/11 CHAPTER 17: Advanced Acid-Base Equilibria Chapter 17 17.1 Acid-Base Reactions 17.2 Buffers 17.3 Acid-Base Titrations Chapter In Context 17.4 Polyprotic Acids We will now expand the introductory coverage of acid-base equilibria in the previous 17.5 Two Important Buffer Systems chapter and explore the chemistry of more complicated aqueous solutions containing acids and bases. First we will address the different types of acid-base reactions and then move on to study buffer solutions, acid-base titrations, and polyprotic acids. So that you can get a feeling for the importance of buffers in your world, we will also briefly discuss Chapter Goals the chemistry of two important buffers in biological systems. In the following chapter Recognize the different we will conclude our coverage of chemical equilibria with Lewis acids and bases and the types of and the extent equilibria of sparingly soluble compounds. of acid-base reactions. Describe the One of the more important types of acid-base solutions in terms of commercial and components of a buffer. biological applications are buffers because they allow us to control the pH of a solution. Apply the principles of Buffers play an important role wherever you look: acid-base equilibria to Biology: You are composed of molecules that depend on hydrogen bonding for their buffer solutions. Apply the concepts of structure and function, and are therefore highly sensitive to pH. Most of the reactions acid-base equilibria to in your body occur in aqueous solutions containing buffering agents. -
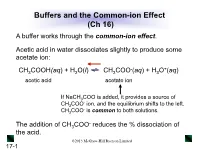
Buffers and the Common-Ion Effect (Ch 16) a Buffer Works Through the Common-Ion Effect
Buffers and the Common-ion Effect (Ch 16) A buffer works through the common-ion effect. Acetic acid in water dissociates slightly to produce some acetate ion: - + CH3COOH(aq) + H2O(l) CH3COO (aq) + H3O (aq) acetic acid acetate ion If NaCH3COO is added, it provides a source of - CH3COO ion, and the equilibrium shifts to the left. - CH3COO is common to both solutions. - The addition of CH3COO reduces the % dissociation of the acid. ©2013 McGraw-Hill Ryerson Limited 17-1 Table 17.1 The Effect of Added Acetate Ion on the Dissociation of Acetic Acid - * + [CH3COOH]init [CH3COO ]added % Dissociation [H3O ] pH 0.10 0.00 1.3 1.3x10-3 2.89 0.10 0.050 0.036 3.6x10-5 4.44 0.10 0.10 0.018 1.8x10-5 4.74 0.10 0.15 0.012 1.2x10-5 4.92 [CH COOH] * % Dissociation = 3 dissoc x 100 [CH3COOH]init ©2013 McGraw-Hill Ryerson Limited 17-2 How a Buffer Works The buffer components (HA and A-) are able to consume - + small amounts of added OH or H3O by a shift in equilibrium position. - + CH3COOH(aq) + H2O(l) CH3COO (aq) + H3O (aq) - + Added OH reacts with Added H3O reacts with - CH3COOH, causing a shift to CH3COO , causing a the right. shift to the left. The shift in equilibrium position absorbs the change in + - [H3O ] or [OH ], and the pH changes only slightly. ©2013 McGraw-Hill Ryerson Limited 17-3 Figure 17.3 How a buffer works. Buffer has more HA after Buffer has equal Buffer has more A- after + - - addition of H3O . -
Buffer Solution Adapted from WIKI
技 术 资 料 集 锦 NO:20140924 Buffer solution in Biology adapted from WIKI A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting Acids and bases of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount ofstrong acid or base is added to it and thus it is used to prevent changes in the pH of a solution. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. Many life forms thrive only in a relatively small pH range so they utilize a buffer solution to maintain a constant pH. One example of a buffer solution found in nature is blood. pH Acid–base reaction Contents Acid–base titration Acid-base extraction Dissociation constant 1 Principles of buffering Acid dissociation constant 2 Applications Acid strength o 2.1 Simple buffering agents Acidity function o 2.2 "Universal" buffer mixtures Buffer solutions o 2.3 Common buffer compounds used in biology Proton affinity 3 Buffer capacity Self-ionization of water 4 Calculating buffer pH Amphoterism o 4.1 Monoprotic acids Acid types o 4.2 Polyprotic acids 5 See also Brønsted 6 References Lewis 7 External links Mineral 0510 81819585 | 189 51576701 | QQ: 2642 166682 | [email protected] 1 无锡天演生物技术有限公司 | 江苏省无锡市智慧路 33 号 7 栋 601 室 技 术 资 料 集 锦 NO:20140924 Organic Strong Superacids Principles of buffering Weak Simulated titration of an acidified solution of a weak acid (pKa = Base types 4.7) with alkali. -

Buffer Preparation and Capacity
Buffer Preparation and Capacity In lab this week you are going to prepare an assigned buffer solution and test the buffering capacity of the solution using strong acid (HCl) and strong base (NaOH). A buffered solution resists changes in pH when acids or bases are added or when dilution occurs. The buffer is a mixture of a weak acid and its conjugate base. The buffering capacity of a solution is determined using the Henderson-Hasselbalch Equation, and the closer the ratio of acid to conjugate base is to one the higher buffering capacity. A buffer also can consist of a weak base and its conjugate acid as well. pH = pKa + log ([A-] / [HA]) where [HA] = concentration of weak acid and [A-] = concentration of the conjugate base Buffer Systems Available in Lab: 1. Tris (2-Amino-2-hydroxymethyl-propane-1,3-diol) pKa = 8.082 OH NH2 HO OH Base only 2. Phosphate Buffer (H3PO4, NaH2PO4·H2O, Na2HPO4, Na3PO4·12H2O) pKa1 = 2.15, pKa2 = 7.20, pKa3 = 12.36 OH HO P OH O Base and Acid 3. Acetate Buffer (HC2H3O2, NaC2H3O2) pKa = 4.76 HO O Base and Acid 4. HEPES (2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid) pKa = 7.5 OH N N SO O OH Other available solutions include: 0.1M HCl, 0.1M NaOH, 6 M HCl, and 6 M NaOH Example calculation for preparing 100.0 mL of 0.1 M phosphate buffer solution at pH = 6.80: (acid and base both available) Now that you have what the concentrations of NaH2PO4·H2O and Na2HPO4 have to be to obtain the desired buffered solution. -

BUFFER SOLUTIONS AP Chemistry
BUFFER SOLUTIONS AP Chemistry Solutions which resist large changes in pH when small amounts of acid or base are added are called buffer solutions. There are two general types of buffers, though they function in similar ways: 1. Acid buffer: a solution containing a weak acid and the conjugate base of the weak acid (for example, HF and NaF) 2. Base buffer: a solution containing a weak base and the conjugate acid of the weak base (for example, NH3 and NH4Cl) Notice each type of buffer contains a species that can function as a weak acid and a species that can function as a weak base. A buffer solution must have each of these components: a weak acid to neutralize additional base and a weak base to neutralize additional acid. Buffer capacity refers to the ability of a buffer system to resist changes in pH. Buffer solutions are most effective when the concentrations of the acid-base pair are about equal. When this is the case, the pH of the buffer solution will be near the pKa for the weak acid. This is important to the buffer’s ability to neutralize either additional acid or base. Buffer capacity is also affected by magnitude of the acid-base concentrations; where the greater the acid-base concentrations, the greater the buffer capacity. For example, a buffer solution of 0.1 M HF and 0.1 M NaF would have a greater buffer capacity than a solution of 0.01 M HF and 0.01 M NaF. This comes down to the number of particles available for neutralization. -

Buffers 1 Buffers
Buffers 1 Buffers • Buffers are exceptionally important in biology. For example, our blood pH is precisely controlled by a buffer involving the − bicarbonate (HCO3 ) ion. − + H2CO3 HCO3 + H A. The Common-Ion Effect • Consider the following ionization of a weak acid: [H+ ][A- ] HA A− + H+ K = a [HA] + • What happens if we added a strong acid (source of H , a common ion) to the solution already in equilibrium? o The reaction quotient Q will be larger than Ka, so the system shifts to the left to re-establish equilibrium. i.e. the presence a common ion = less HA ionization • Example: What is the pH of a solution containing 0.20 M −5 acetic acid (Ka = 1.8 × 10 ) and 0.05 M HCl? What is the % ionization of acetic acid? Buffers 2 B. Buffers • In the last example, we added a strong acid and a weak acid together (the common ion was H+). • What if we had a solution of approx equal amounts of both weak acid (HA) and its conjugate base (as salt, e.g. NaA)? • Under these circumstances, we could have a buffer solution. Buffers are solutions that are able to resist drastic changes in pH upon the addition of strong acid or base. HA A− + H+ o If we have enough A−, the reaction could use up any strong acid that is added (by shifting to the left). If we have enough HA, it could shift to the right and replace the H+ that is used by the addition of strong base Buffers 3 • Buffers therefore must have both the weak acid and its conjugate base (or a weak base and its conjugate acid). -

Safety Data Sheet Buffer Solution Ph 10 DANGER
Safety Data Sheet Buffer Solution pH 10 Section 1 Product Description Product Name: Buffer Solution pH 10 Recommended Use: Science education applications Synonyms: None known Distributor: Carolina Biological Supply Company 2700 York Road, Burlington, NC 27215 1-800-227-1150 Chemical Information: 800-227-1150 (8am-5pm (ET) M-F) Chemtrec: 800-424-9300 (Transportation Spill Response 24 hours) Section 2 Hazard Identification Classification of the chemical in accordance with paragraph (d) of §1910.1200; DANGER May damage fertility or the unborn child. GHS Classification: Reproductive Toxicity Category 1B Other Safety Precautions: IF exposed or concerned: Get medical advice/attention. Section 3 Composition / Information on Ingredients Chemical Name CAS # % Water 7732-18-5 99.08 Potassium Chloride 7447-40-7 0.4 Boric Acid 10043-35-3 0.33 Sodium Hydroxide 1310-73-2 0.19 Section 4 First Aid Measures Emergency and First Aid Procedures Inhalation: In case of accident by inhalation: remove casualty to fresh air and keep at rest. Eyes: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Skin Contact: After contact with skin, wash immediately with plenty of water. Ingestion: If swallowed, do not induce vomiting: seek medical advice immediately and show this container or label. Section 5 Firefighting Procedures Extinguishing Media: Use media suitable to extinguish surrounding fire. Fire Fighting Methods and Protection: Firefighters should wear full protective equipment and NIOSH approved self-contained breathing apparatus. Fire and/or Explosion Hazards: Fire or excessive heat may produce hazardous decomposition products. Hazardous Combustion Products: Boron Compounds, Sodium Oxides Section 6 Spill or Leak Procedures Buffer Solution pH 10 Page 1 of 4 Safety Data Sheet Steps to Take in Case Material Is Exposure to the spilled material may be irritating or harmful.