Sociation Constant of the Buffer and the Concentration and Reaction of the Buffer Solution.*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Choosing Buffers Based on Pka in Many Experiments, We Need a Buffer That Maintains the Solution at a Specific Ph. We May Need An
Choosing buffers based on pKa In many experiments, we need a buffer that maintains the solution at a specific pH. We may need an acidic or a basic buffer, depending on the experiment. The Henderson-Hasselbalch equation can help us choose a buffer that has the pH we want. pH = pKa + log([conj. base]/[conj. acid]) With equal amounts of conjugate acid and base (preferred so buffers can resist base and acid equally), then … pH = pKa + log(1) = pKa + 0 = pKa So choose conjugates with a pKa closest to our target pH. Chemistry 103 Spring 2011 Example: You need a buffer with pH of 7.80. Which conjugate acid-base pair should you use, and what is the molar ratio of its components? 2 Chemistry 103 Spring 2011 Practice: Choose the best conjugate acid-base pair for preparing a buffer with pH 5.00. What is the molar ratio of the buffer components? 3 Chemistry 103 Spring 2011 buffer capacity: the amount of strong acid or strong base that can be added to a buffer without changing its pH by more than 1 unit; essentially the number of moles of strong acid or strong base that uses up all of the buffer’s conjugate base or conjugate acid. Example: What is the capacity of the buffer solution prepared with 0.15 mol lactic acid -4 CH3CHOHCOOH (HA, Ka = 1.0 x 10 ) and 0.20 mol sodium lactate NaCH3CHOHCOO (NaA) and enough water to make 1.00 L of solution? (from previous lecture notes) 4 Chemistry 103 Spring 2011 Review of equivalence point equivalence point: moles of H+ = moles of OH- (moles of acid = moles of base, only when the acid has only one acidic proton and the base has only one hydroxide ion). -

Buffers a Guide for the Preparation and Use of Buffers in Biological Systems Calbiochem® Buffers a Guide for the Preparation and Use of Buffers in Biological Systems
Buffers A guide for the preparation and use of buffers in biological systems Calbiochem® Buffers A guide for the preparation and use of buffers in biological systems Chandra Mohan, Ph.D. EMD, San Diego, California © EMD, an affiliate of Merck KGaA, Darmstadt, Germany. All rights reserved. A word to our valued customers We are pleased to present to you the newest edition of Buffers: A Guide for the Preparation and Use of Buffers in Biological Systems. This practical resource has been especially revamped for use by researchers in the biological sciences. This publication is a part of our continuing commitment to provide useful product information and exceptional service to you, our customers. You will find this booklet a highly useful resource, whether you are just beginning your research work or training the newest researchers in your laboratory. Over the past several years, EMD Biosciences has clearly emerged as a world leader in providing highly innovative products for your research needs in Signal Transduction, including the areas of Cancer Biology, Alzheimer’s Disease, Diabetes, Hypertension, Inflammation, and Apoptosis. Please call us today for a free copy of our LATEST Catalog that includes tools for signal transduction and life science research. If you have used our products in the past, we thank you for your support and confidence in our products, and if you are just beginning your research career, please call us and give us the opportunity to demonstrate our exceptional customer and technical service. Corrine Fetherston Sr. Director, Marketing ii Table of Contents: Why does Calbiochem® Biochemicals Publish a Booklet on Buffers? . -

Absolute Measurement of the Abundance of Atmospheric Carbon Monoxide C
Absolute measurement of the abundance of atmospheric carbon monoxide C. Brenninkmeijer, C. Koeppel, T. Röckmann, D. Scharffe, Maya Bräunlich, Valerie Gros To cite this version: C. Brenninkmeijer, C. Koeppel, T. Röckmann, D. Scharffe, Maya Bräunlich, et al.. Absolute mea- surement of the abundance of atmospheric carbon monoxide. Journal of Geophysical Research: At- mospheres, American Geophysical Union, 2001, 106 (D9), pp.10003-10010. 10.1029/2000JD900342. hal-03117189 HAL Id: hal-03117189 https://hal.archives-ouvertes.fr/hal-03117189 Submitted on 8 Feb 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 106, NO. D9, PAGES 10,003-10,010, MAY 16, 2001 Absolute measurement of the abundance of atmospheric carbon monoxide C. A.M. Brenninkmeijer,C. Koeppel,T. R6ckmann,D. S. Scharffe, Maya Br•iunlich,and Valerie Gros AtmosphericChemistry Division, Max PlanckInstitute for Chemistry,Mainz, Germany Abstract.The main aspects of anabsolute method for measurementof the mixing ratio of atmos- phericcarbon monoxide (CO) are presented. The method is based on cryogenic extraction of CO fromair afteroxidation to CO2followed by accuratevolumetric determination. Gravimetric meas- urementis usedto determinethe quantity of sampleair processed.In routineoperation the overall errorcan be kept below 1%. -

AMMONIA BUFFER SOLUTION MSDS CAS-No
AMMONIA BUFFER SOLUTION MSDS CAS-No.: MSDS MATERIAL SAFETY DATA SHEET (MSDS) SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Mixture : Product code : 01077 1.2. Relevant identified uses of the substance or mixture and uses advised against 1.2.1. Relevant identified uses Use of the substance/mixture : Laboratory chemicals, Manufacture of substances 1.2.2. Uses advised against No additional information available 1.3. Details of the supplier of the safety data sheet LOBA CHEMIE PVT.LTD. 107 Wode House Road, Jehangir Villa, Colaba 400005 Mumbai - INDIA T +91 22 6663 6663 - F +91 22 6663 6699 [email protected] - www.lobachemie.com 1.4. Emergency telephone number Emergency number : + 91 22 6663 6663 (9:00am - 6:00 pm) SECTION 2: Hazards identification 2.1. Classification of the substance or mixture Classification according to Regulation (EC) No. 1272/2008 [CLP]Mixtures/Substances: SDS EU 2015: According to Regulation (EU) 2015/830 (REACH Annex II) Skin corrosion/irritation, H314 Category 1B Specific target organ H335 toxicity — Single exposure, Category 3, Respiratory tract irritation Hazardous to the aquatic H400 environment — Acute Hazard, Category 1 Full text of hazard classes and H-statements : see section 16 Adverse physicochemical, human health and environmental effects No additional information available www.lobachemie.com 21/04/2016 1/9 AMMONIA BUFFER SOLUTION Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH) with its amendment Regulation (EU) 2015/830 2.2. Label elements Labelling according to Regulation (EC) No. 1272/2008 [CLP] Extra labelling to displayExtra classification(s) to display Hazard pictograms (CLP) : GHS05 GHS07 GHS09 Signal word (CLP) : Danger Hazard statements (CLP) : H314 - Causes severe skin burns and eye damage. -

Effects on Accuracy and Detection Limits
WHITE Sensitivity, Background, Noise, and Calibration in Atomic PAPER Spectroscopy: Effects on Accuracy and Detection Limits Introduction Proper calibration in atomic spectroscopy and an understanding The three most common atomic spectroscopy techniques of uncertainty is fundamental to achieving accurate results. This are atomic absorption (AA) spectroscopy, ICP optical emission paper provides a practical discussion of the effects of noise, error spectroscopy (ICP-OES) and ICP mass spectrometry (ICP-MS). and concentration range of calibration curves on the ability to Of these, ICP-OES and ICP-MS are very linear; that is, a plot determine the concentration of a given element with reasonable of concentration vs. intensity forms a straight line over a accuracy. The determination of lower limits of quantitation and wide range of concentrations (Figure 1). AA is linear over a lower limits of detection will also be discussed. much smaller range and begins to curve downward at higher Results accuracy is highly dependent on blank contamination, concentrations (Figure 2). Linear ranges are well understood, linearity of calibration standards, curve-fitting choices and the and, for AA, a rule of thumb can be applied to estimate the range of concentrations chosen for calibration. Additional factors maximum working range using a non-linear algorithm. include the use of internal standards (and proper selection of This paper will discuss the contributions of sensitivity, internal standards) and instrumental settings. background, noise, calibration range, calibration mathematics This paper is not intended to be a rigorous treatment of statistics and contamination on the ability to achieve best accuracy and in calibration; many references are available for this, such as lowest detection limits. -

Combined Wastestream Formula (CWF)
UnitedStates PermitsDivision and September1985 EnvironmentalProtection IndustrialTechnology Division Agency Washington,DC 20460 Water EPA Guidance Manual Pretreatment for the Use of Production-Based Pretreatment Standards and the Combined Wastestream Formula UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 SEP 19 1985 OFFICEOF WATER MEMORANDUM SUBJECT: Pretreatment Program Guidance FROM: Rebecca W. Hanmer, Director Office of Water Enforcement and Permits (EN-335) James M. Conlon, Acting Director Office of Water Regulations and Standards (WH-551) TO: Users of the Guidance Manual for the Use of Production- Based Categorical Pretreatment Standards and the Combined Wastestream Formula This guidance manual has been developed by EPA to explain how to implement two important elements of the national pretreatment program: categorical standards and the combined wastestream formula. The manual is divided into two sections. The first section explains how to apply production-based categorical standards in a permit, contract, or similar mechanism. The second part explains how to use the combined wastestream formula, providing definitions and examples. The manual is one of a series of guidance documents intended to simplify and improve understanding of various aspects of the pretreatment program. Other documents in this series which have either been recently issued or will be issued in the near future will provide guidance on: 1) Removal Credits 2) Total Toxic Organics (TTO) Monitoring 3) RCRA Notification Requirements 4) Local Limits 5) POTW Interference The need for guidance on the use of categorical standards and the combined wastestream formula was recognized by the Pretreatment Implementation Review Task Force (PIRT). PIRT was set up by the EPA Administrator to make recommendations concerning the problems faced by POTWs, states, and industry in implementing the national pretreatment program. -

Solvent Effects on the Thermodynamic Functions of Dissociation of Anilines and Phenols
University of Wollongong Research Online University of Wollongong Thesis Collection 1954-2016 University of Wollongong Thesis Collections 1982 Solvent effects on the thermodynamic functions of dissociation of anilines and phenols Barkat A. Khawaja University of Wollongong Follow this and additional works at: https://ro.uow.edu.au/theses University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Recommended Citation Khawaja, Barkat A., Solvent effects on the thermodynamic functions of dissociation of anilines and phenols, Master of Science thesis, Department of Chemistry, University of Wollongong, 1982. -

3.1 Polarization Behavior of Active Passive Metals and Alloys Glossary
3.1 Polarization Behavior of Active Passive Metals and Alloys John R. Scully, Katie Lutton Center for Electrochemical Science and Engineering Department of Materials Science and Engineering University of Virginia, Charlottesville, VA, United States Glossary Symbol Explanation 퐸푃 Primary passivation potential 퐸푃2 Primary activation potential 푖푐푟푖푡 Critical current density 퐸푡푟푎푛푠 Transpassive potential 퐸푃1 First primary passivation potential 표 퐸푃 Hypothetical standard primary passivation potential based on a half-cell reaction 휏푃 Critical time for passivation 푆퐻퐸 Standard hydrogen electrode 푖푝푎푠푠 Passive current density 푖lim Limiting current density for a mass transport limited cathodic reaction Abstract The objective of this chapter is to summarize the relationships between electrochemical potential and current density for typical transition metals such as Fe, Cr, and Ni. Key parameters such as the active passive transition, primary and secondary passivation potentials, critical anodic current density, passive current density, and transpassive region are presented, defined, and interpreted to explain typical E-log(i) diagrams and polarization curves. Consideration is given to the polarization behavior of a passivating electrode resulting from both the anodic and cathodic partial currents using mixed potential theory. The impact of the reversible electrode potential and polarizability of an oxidizing reducible species on the observed E-log(i) polarization behavior of such passivated materials is discussed. The kinetics of the passive film -

Buffers and Buffer Capacity
BUFFERS AND BUFFER CAPACITY By Dr. Jan Pláteník Principle of buffering A buffer solution is a solution that resists changes in pH either when diluted or when limited amounts of acid or base are added to it. Such a solution can be prepared by combining a weak acid and its salt with a strong base (conjugated base) or, analogously, a weak base and its salt with a strong acid (conjugated acid). For example: Acetate buffer : CH 3COOH (the weak acid) + CH 3COONa (the salt, conjugated base) Phosphate buffer : NaH 2PO 4 (the weak acid) + Na 2HPO 4 (the salt, conjugated base) Tris buffer: (Tris: Tris [2-amino-2-(hydroxymethyl)-propan-1,3-diol)] , an organic base) The Henderson-Hasselbalch equation describes the behavior of such a buffer and for the mixture of a weak acid and its salt with a strong base (conjugated base) it has the form: cs pH = pK a + log cac pK a negative logarithm of the dissociation constant for the weak acid, cs substance concentration of the salt (conjugated base), cac substance concentration of the weak acid (conjugated acid). Graphically the Henderson-Hasselbalch equation plotted as the acid : conjugated base ratio vs. pH of buffer actually constitutes the titration curve of the weak acid (see figure on the next page). Note also that for the acid : base ratio 1:1 the pH of buffer just equals the pK a (this is valid for uni-univalent systems, e.g., CH 3COOH/CH 3COONa, NaH 2PO 4/Na 2HPO 4). 1 Titration curve of sodium phosphate buffer 10 9 8 pH = pKa = 7.21 7 pHof buffer 6 5 9:1 8:2 7:3 6:4 1:1 4:6 3:7 2:8 1:9 Acid : base ratio The equation for a weak base and its salt with a strong acid (conjugated acid) has the form: cb pH = pK w − pK b + log cs pK b negative logarithm of the dissociation constant for the weak base, cb substance concentration of the base, cs substance concentration of the salt (conjugated acid), -14 pK w = 14 = − log 10 (ionic product of water). -

CHAPTER 17: Advanced Acid-Base Equilibria
Chapter 17 Advanced Acid-Base Equilibria SY 4/12/11 CHAPTER 17: Advanced Acid-Base Equilibria Chapter 17 17.1 Acid-Base Reactions 17.2 Buffers 17.3 Acid-Base Titrations Chapter In Context 17.4 Polyprotic Acids We will now expand the introductory coverage of acid-base equilibria in the previous 17.5 Two Important Buffer Systems chapter and explore the chemistry of more complicated aqueous solutions containing acids and bases. First we will address the different types of acid-base reactions and then move on to study buffer solutions, acid-base titrations, and polyprotic acids. So that you can get a feeling for the importance of buffers in your world, we will also briefly discuss Chapter Goals the chemistry of two important buffers in biological systems. In the following chapter Recognize the different we will conclude our coverage of chemical equilibria with Lewis acids and bases and the types of and the extent equilibria of sparingly soluble compounds. of acid-base reactions. Describe the One of the more important types of acid-base solutions in terms of commercial and components of a buffer. biological applications are buffers because they allow us to control the pH of a solution. Apply the principles of Buffers play an important role wherever you look: acid-base equilibria to Biology: You are composed of molecules that depend on hydrogen bonding for their buffer solutions. Apply the concepts of structure and function, and are therefore highly sensitive to pH. Most of the reactions acid-base equilibria to in your body occur in aqueous solutions containing buffering agents. -

Florpyrauxifen-Benzyl & Degradates in Compost
Page 16 INTRODUCTION Scope The objective of the study was to independently validate analytical method as given in the DAS study 171407 [1] for the determination of Florpyrauxifen benzyl (XDE-848 BE), X11438848 and X11966341 in compost in accordance to the guidance documents SANCO/825/00, rev. 8.1 [2] and SANCO/3029/99 rev. 4 of the European Commission [3], and OCSPP 850.6100 of the United States Environmental Protection Agency [4]. The limit of quantification was 0.00015 mg/kg for Florpyrauxifen benzyl (XDE-848 BE), 0.00045 mg/kg for X11438848 and 0.009 mg/kg for X11966341 Analytical Procedure Compost samples were extracted by shaking with acetonitrile/0.1N hydrochloric acid (90:10, v/v), centrifuging, and decanting into a separate tube containing QuEChERS Citrat kit. An aliquot of 1N HCl was added to the extract followed by centrifugation. After a portion of the organic layer was aliquoted, internal standard was added and the sample was evaporated to near dryness under a stream of nitrogen. 1N HCl was added and the samples were incubated for one hour at 80 °C. Ethyl acetate was added and the samples were transferred to a Supel QuE Z-Sep tube and centrifuged. After centrifuging, the samples were placed in a dry ice bath to flash freeze the aqueous layer and the organic layer was poured off into a glass tube. The samples were evaporated under a stream of nitrogen, reconstituted in methanol and 0.1% formic acid in water, and transferred into an autosampler vial for analysis. The samples were analyzed for XDE-848 BE and its metabolites by liquid chromatography with positive ion electrospray ionization tandem mass spectrometry Selectivity Quantification was performed by use of LC-MS/MS detection. -
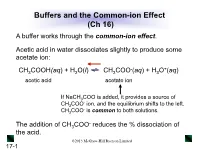
Buffers and the Common-Ion Effect (Ch 16) a Buffer Works Through the Common-Ion Effect
Buffers and the Common-ion Effect (Ch 16) A buffer works through the common-ion effect. Acetic acid in water dissociates slightly to produce some acetate ion: - + CH3COOH(aq) + H2O(l) CH3COO (aq) + H3O (aq) acetic acid acetate ion If NaCH3COO is added, it provides a source of - CH3COO ion, and the equilibrium shifts to the left. - CH3COO is common to both solutions. - The addition of CH3COO reduces the % dissociation of the acid. ©2013 McGraw-Hill Ryerson Limited 17-1 Table 17.1 The Effect of Added Acetate Ion on the Dissociation of Acetic Acid - * + [CH3COOH]init [CH3COO ]added % Dissociation [H3O ] pH 0.10 0.00 1.3 1.3x10-3 2.89 0.10 0.050 0.036 3.6x10-5 4.44 0.10 0.10 0.018 1.8x10-5 4.74 0.10 0.15 0.012 1.2x10-5 4.92 [CH COOH] * % Dissociation = 3 dissoc x 100 [CH3COOH]init ©2013 McGraw-Hill Ryerson Limited 17-2 How a Buffer Works The buffer components (HA and A-) are able to consume - + small amounts of added OH or H3O by a shift in equilibrium position. - + CH3COOH(aq) + H2O(l) CH3COO (aq) + H3O (aq) - + Added OH reacts with Added H3O reacts with - CH3COOH, causing a shift to CH3COO , causing a the right. shift to the left. The shift in equilibrium position absorbs the change in + - [H3O ] or [OH ], and the pH changes only slightly. ©2013 McGraw-Hill Ryerson Limited 17-3 Figure 17.3 How a buffer works. Buffer has more HA after Buffer has equal Buffer has more A- after + - - addition of H3O .