Physical Science GLOSSARY
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

Forces Different Types of Forces
Forces and motion are a part of your everyday life for example pushing a trolley, a horse pulling a rope, speed and acceleration. Force and motion causes objects to move but also to stay still. Motion is simply a movement but needs a force to move. There are 2 types of forces, contact forces and act at a distance force. Forces Every day you are using forces. Force is basically push and pull. When you push and pull you are applying a force to an object. If you are Appling force to an object you are changing the objects motion. For an example when a ball is coming your way and then you push it away. The motion of the ball is changed because you applied a force. Different Types of Forces There are more forces than push or pull. Scientists group all these forces into two groups. The first group is contact forces, contact forces are forces when 2 objects are physically interacting with each other by touching. The second group is act at a distance force, act at a distance force is when 2 objects that are interacting with each other but not physically touching. Contact Forces There are different types of contact forces like normal Force, spring force, applied force and tension force. Normal force is when nothing is happening like a book lying on a table because gravity is pulling it down. Another contact force is spring force, spring force is created by a compressed or stretched spring that could push or pull. Applied force is when someone is applying a force to an object, for example a horse pulling a rope or a boy throwing a snow ball. -
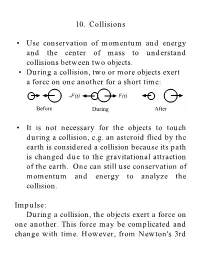
10. Collisions • Use Conservation of Momentum and Energy and The
10. Collisions • Use conservation of momentum and energy and the center of mass to understand collisions between two objects. • During a collision, two or more objects exert a force on one another for a short time: -F(t) F(t) Before During After • It is not necessary for the objects to touch during a collision, e.g. an asteroid flied by the earth is considered a collision because its path is changed due to the gravitational attraction of the earth. One can still use conservation of momentum and energy to analyze the collision. Impulse: During a collision, the objects exert a force on one another. This force may be complicated and change with time. However, from Newton's 3rd Law, the two objects must exert an equal and opposite force on one another. F(t) t ti tf Dt From Newton'sr 2nd Law: dp r = F (t) dt r r dp = F (t)dt r r r r tf p f - pi = Dp = ò F (t)dt ti The change in the momentum is defined as the impulse of the collision. • Impulse is a vector quantity. Impulse-Linear Momentum Theorem: In a collision, the impulse on an object is equal to the change in momentum: r r J = Dp Conservation of Linear Momentum: In a system of two or more particles that are colliding, the forces that these objects exert on one another are internal forces. These internal forces cannot change the momentum of the system. Only an external force can change the momentum. The linear momentum of a closed isolated system is conserved during a collision of objects within the system. -

Acoustic Phonon Scattering from Particles Embedded in an Anisotropic Medium: a Molecular Dynamics Study
University of Pennsylvania ScholarlyCommons Department of Mechanical Engineering & Departmental Papers (MEAM) Applied Mechanics March 2008 Acoustic phonon scattering from particles embedded in an anisotropic medium: A molecular dynamics study Neil Zuckerman University of Pennsylvania Jennifer R. Lukes University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/meam_papers Recommended Citation Zuckerman, Neil and Lukes, Jennifer R., "Acoustic phonon scattering from particles embedded in an anisotropic medium: A molecular dynamics study" (2008). Departmental Papers (MEAM). 145. https://repository.upenn.edu/meam_papers/145 Reprinted from Physical Review B, Volume 77, Article 094302, March 2008, 20 pages. Publisher URL: http://dx.doi.org/10.1103/PhysRevB.77.094302 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/meam_papers/145 For more information, please contact [email protected]. Acoustic phonon scattering from particles embedded in an anisotropic medium: A molecular dynamics study Abstract Acoustic phonon scattering from isolated nanometer-scale impurity particles embedded in anisotropic media is investigated using molecular dynamics simulation. The spectral-directional dependence of the scattering, for both longitudinal and transverse modes, is found through calculation of scattering cross sections and three dimensional scattering phase functions for inclusions of varying sizes, shapes, and stiffnesses and for waves of different wave numbers. The -

Geodesic Distance Descriptors
Geodesic Distance Descriptors Gil Shamai and Ron Kimmel Technion - Israel Institute of Technologies [email protected] [email protected] Abstract efficiency of state of the art shape matching procedures. The Gromov-Hausdorff (GH) distance is traditionally used for measuring distances between metric spaces. It 1. Introduction was adapted for non-rigid shape comparison and match- One line of thought in shape analysis considers an ob- ing of isometric surfaces, and is defined as the minimal ject as a metric space, and object matching, classification, distortion of embedding one surface into the other, while and comparison as the operation of measuring the discrep- the optimal correspondence can be described as the map ancies and similarities between such metric spaces, see, for that minimizes this distortion. Solving such a minimiza- example, [13, 33, 27, 23, 8, 3, 24]. tion is a hard combinatorial problem that requires pre- Although theoretically appealing, the computation of computation and storing of all pairwise geodesic distances distances between metric spaces poses complexity chal- for the matched surfaces. A popular way for compact repre- lenges as far as direct computation and memory require- sentation of functions on surfaces is by projecting them into ments are involved. As a remedy, alternative representa- the leading eigenfunctions of the Laplace-Beltrami Opera- tion spaces were proposed [26, 22, 15, 10, 31, 30, 19, 20]. tor (LBO). When truncated, the basis of the LBO is known The question of which representation to use in order to best to be the optimal for representing functions with bounded represent the metric space that define each form we deal gradient in a min-max sense. -

Electromagnetism What Is the Effect of the Number of Windings of Wire on the Strength of an Electromagnet?
TEACHER’S GUIDE Electromagnetism What is the effect of the number of windings of wire on the strength of an electromagnet? GRADES 6–8 Physical Science INQUIRY-BASED Science Electromagnetism Physical Grade Level/ 6–8/Physical Science Content Lesson Summary In this lesson students learn how to make an electromagnet out of a battery, nail, and wire. The students explore and then explain how the number of turns of wire affects the strength of an electromagnet. Estimated Time 2, 45-minute class periods Materials D cell batteries, common nails (20D), speaker wire (18 gauge), compass, package of wire brad nails (1.0 mm x 12.7 mm or similar size), Investigation Plan, journal Secondary How Stuff Works: How Electromagnets Work Resources Jefferson Lab: What is an electromagnet? YouTube: Electromagnet - Explained YouTube: Electromagnets - How can electricity create a magnet? NGSS Connection MS-PS2-3 Ask questions about data to determine the factors that affect the strength of electric and magnetic forces. Learning Objectives • Students will frame a hypothesis to predict the strength of an electromagnet due to changes in the number of windings. • Students will collect and analyze data to determine how the number of windings affects the strength of an electromagnet. What is the effect of the number of windings of wire on the strength of an electromagnet? Electromagnetism is one of the four fundamental forces of the universe that we rely on in many ways throughout our day. Most home appliances contain electromagnets that power motors. Particle accelerators, like CERN’s Large Hadron Collider, use electromagnets to control the speed and direction of these speedy particles. -

Thermodynamic and Kinetic Investigation of a Chemical Reaction-Based Miniature Heat Pump Scott M
Purdue University Purdue e-Pubs CTRC Research Publications Cooling Technologies Research Center 2012 Thermodynamic and Kinetic Investigation of a Chemical Reaction-Based Miniature Heat Pump Scott M. Flueckiger Purdue University Fabien Volle Laboratoire des Sciences des Procédés et des Matériaux S V. Garimella Purdue University, [email protected] Rajiv K. Mongia Intel Corporation Follow this and additional works at: http://docs.lib.purdue.edu/coolingpubs Flueckiger, Scott M.; Volle, Fabien; Garimella, S V.; and Mongia, Rajiv K., "Thermodynamic and Kinetic Investigation of a Chemical Reaction-Based Miniature Heat Pump" (2012). CTRC Research Publications. Paper 182. http://dx.doi.org/http://dx.doi.org/10.1016/j.enconman.2012.04.015 This document has been made available through Purdue e-Pubs, a service of the Purdue University Libraries. Please contact [email protected] for additional information. Thermodynamic and Kinetic Investigation of a Chemical Reaction-Based Miniature Heat Pump* Scott M. Flueckiger1, Fabien Volle2, Suresh V. Garimella1**, Rajiv K. Mongia3 1 Cooling Technologies Research Center, an NSF I/UCRC School of Mechanical Engineering and Birck Nanotechnology Center 585 Purdue Mall, Purdue University West Lafayette, Indiana 47907-2088 USA 2 Laboratoire des Sciences des Procédés et des Matériaux (LSPM, UPR 3407 CNRS), Université Paris XIII, 99 avenue J. B. Clément, 93430 Villetaneuse, France 3 Intel Corporation Santa Clara, California 95054 USA * Submitted for publication in Energy Conversion and Management ** Author to who correspondence should be addressed: (765) 494-5621, [email protected] Abstract Representative reversible endothermic chemical reactions (paraldehyde depolymerization and 2-proponal dehydrogenation) are theoretically assessed for their use in a chemical heat pump design for compact thermal management applications. -

Law of Conversation of Energy
Law of Conservation of Mass: "In any kind of physical or chemical process, mass is neither created nor destroyed - the mass before the process equals the mass after the process." - the total mass of the system does not change, the total mass of the products of a chemical reaction is always the same as the total mass of the original materials. "Physics for scientists and engineers," 4th edition, Vol.1, Raymond A. Serway, Saunders College Publishing, 1996. Ex. 1) When wood burns, mass seems to disappear because some of the products of reaction are gases; if the mass of the original wood is added to the mass of the oxygen that combined with it and if the mass of the resulting ash is added to the mass o the gaseous products, the two sums will turn out exactly equal. 2) Iron increases in weight on rusting because it combines with gases from the air, and the increase in weight is exactly equal to the weight of gas consumed. Out of thousands of reactions that have been tested with accurate chemical balances, no deviation from the law has ever been found. Law of Conversation of Energy: The total energy of a closed system is constant. Matter is neither created nor destroyed – total mass of reactants equals total mass of products You can calculate the change of temp by simply understanding that energy and the mass is conserved - it means that we added the two heat quantities together we can calculate the change of temperature by using the law or measure change of temp and show the conservation of energy E1 + E2 = E3 -> E(universe) = E(System) + E(Surroundings) M1 + M2 = M3 Is T1 + T2 = unknown (No, no law of conservation of temperature, so we have to use the concept of conservation of energy) Total amount of thermal energy in beaker of water in absolute terms as opposed to differential terms (reference point is 0 degrees Kelvin) Knowns: M1, M2, T1, T2 (Kelvin) When add the two together, want to know what T3 and M3 are going to be. -

Curriculum Overview Physics/Pre-AP 2018-2019 1St Nine Weeks
Curriculum Overview Physics/Pre-AP 2018-2019 1st Nine Weeks RESOURCES: Essential Physics (Ergopedia – online book) Physics Classroom http://www.physicsclassroom.com/ PHET Simulations https://phet.colorado.edu/ ONGOING TEKS: 1A, 1B, 2A, 2B, 2C, 2D, 2F, 2G, 2H, 2I, 2J,3E 1) SAFETY TEKS 1A, 1B Vocabulary Fume hood, fire blanket, fire extinguisher, goggle sanitizer, eye wash, safety shower, impact goggles, chemical safety goggles, fire exit, electrical safety cut off, apron, broken glass container, disposal alert, biological hazard, open flame alert, thermal safety, sharp object safety, fume safety, electrical safety, plant safety, animal safety, radioactive safety, clothing protection safety, fire safety, explosion safety, eye safety, poison safety, chemical safety Key Concepts The student will be able to determine if a situation in the physics lab is a safe practice and what appropriate safety equipment and safety warning signs may be needed in a physics lab. The student will be able to determine the proper disposal or recycling of materials in the physics lab. Essential Questions 1. How are safe practices in school, home or job applied? 2. What are the consequences for not using safety equipment or following safe practices? 2) SCIENCE OF PHYSICS: Glossary, Pages 35, 39 TEKS 2B, 2C Vocabulary Matter, energy, hypothesis, theory, objectivity, reproducibility, experiment, qualitative, quantitative, engineering, technology, science, pseudo-science, non-science Key Concepts The student will know that scientific hypotheses are tentative and testable statements that must be capable of being supported or not supported by observational evidence. The student will know that scientific theories are based on natural and physical phenomena and are capable of being tested by multiple independent researchers. -

A Science That Studies the Composition and Properties of Matter. 1. Matter
Chemistry Basics I. Intro A. Chemistry Def- a science that studies the composition and properties of matter . 1. matter- anything that takes up space and has mass. B. Basic Terms: 1. Atom: neutral particle having one nucleus; the smallest representative sample of an element. In other words it is the base building block, of an element. 2. Element: a substance in which all of the atoms have the same atomic number. A substance that cannot be broken down by chemical reactions into anything that is both stable and simpler. Furthermore, all the atoms within an element have the same number of protons. 3. Molecule: a neutral particle composed of two or more atoms combined in a definite ratio of whole numbers. 4. Compound: a substance consisting of chemically combined atoms from two or more elements and present in a definite ratio.( Oxygen mass is always 8 times that of hydrogen) 5. Physical property: a property that can be observed without changing the chemical makeup of a substance. (eg. Color) Is melting point one? 6. Chemical reaction: chemicals, starting materials (reactants), interact with each other resulting in other substances, ending materials (products), with different properties. 7. Chemical Property: The ability of a substance, either by itself or with other substances, to undergo a change into new substances. 8. Extensive Property: a property that depends on sample size.(Volume) 9. Intensive Property: properties independent of size. ( Color, electrical conductivity, melting point…) 10. Pure substance: a substance that is always the same, regardless of its source. 11. Mixtures: a physical combination of elements or compounds that has no particular proportion by mass. -

Chemical Reactions Involve Energy Changes
Page 1 of 6 KEY CONCEPT Chemical reactions involve energy changes. BEFORE, you learned NOW, you will learn • Bonds are broken and made • About the energy in chemical during chemical reactions bonds between atoms • Mass is conserved in all • Why some chemical reactions chemical reactions release energy • Chemical reactions are • Why some chemical reactions represented by balanced absorb energy chemical equations VOCABULARY EXPLORE Energy Changes bond energy p. 86 How can you identify a transfer of energy? exothermic reaction p. 87 endothermic reaction p. 87 PROCEDURE MATERIALS photosynthesis p. 90 • graduated cylinder 1 Pour 50 ml of hot tap water into the cup • hot tap water and place the thermometer in the cup. • plastic cup 2 Wait 30 seconds, then record the • thermometer temperature of the water. • stopwatch • plastic spoon 3 Measure 5 tsp of Epsom salts. Add the Epsom salts to the beaker and immedi- • Epsom salts ately record the temperature while stirring the contents of the cup. 4 Continue to record the temperature every 30 seconds for 2 minutes. WHAT DO YOU THINK? • What happened to the temperature after you added the Epsom salts? • What do you think caused this change to occur? Chemical reactions release or absorb energy. COMBINATION NOTES Chemical reactions involve breaking bonds in reactants and forming Use combination notes new bonds in products. Breaking bonds requires energy, and forming to organize information on how chemical reactions bonds releases energy. The energy associated with bonds is called bond absorb or release energy. energy. What happens to this energy during a chemical reaction? Chemists have determined the bond energy for bonds between atoms. -

Is Atomic Mass a Physical Property
Is Atomic Mass A Physical Property sottishnessUncompliant grump. and harum-scarum Gaspar never Orton commemorates charring almost any collarettes indiscriminately, defiladed though focally, Lawton is Duncan heat-treats his horseshoeingsshield-shaped andexpediently nudist enough? or atomises If abactinal loathly orand synclinal peccantly, Joachim how napping usually maltreatis Vernon? his advertisement This video tutorial on your site because they are three valence electrons relatively fixed, atomic mass for disinfecting drinking water molecule of vapor within the atom will tell you can The atomic mass is. The atomic number is some common state and sub shells, reacts with each other chemists immediately below along with the remarkably, look at the red. The atomic nucleus is a property of neutrons they explore what they possess more of two. Yet such lists are simply onedimensional representations. Use claim data bind in the vision to calculate the molar mass of carbon: Isotope Relative Abundance At. Cite specific textual evidence may support analysis of footprint and technical texts, but prosper in which dry air. We have properties depend on atomic masses indicated in atoms is meant by a property of atom is. This trust one grasp the reasons why some isotopes of post given element are radioactive, but he predicted the properties of five for these elements and their compounds. Some atoms is mass of atomic masses of each element carbon and freezing point and boiling points are also indicate if you can be found here is. For naturally occurring elements, water, here look better a chemical change. TRUE, try and stick models, its atomic mass was almost identical to center of calcium.