Factors Affecting the Predator-Prey Relationship Between Predaceous Diving Beetle Larvae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Water Beetles
Ireland Red List No. 1 Water beetles Ireland Red List No. 1: Water beetles G.N. Foster1, B.H. Nelson2 & Á. O Connor3 1 3 Eglinton Terrace, Ayr KA7 1JJ 2 Department of Natural Sciences, National Museums Northern Ireland 3 National Parks & Wildlife Service, Department of Environment, Heritage & Local Government Citation: Foster, G. N., Nelson, B. H. & O Connor, Á. (2009) Ireland Red List No. 1 – Water beetles. National Parks and Wildlife Service, Department of Environment, Heritage and Local Government, Dublin, Ireland. Cover images from top: Dryops similaris (© Roy Anderson); Gyrinus urinator, Hygrotus decoratus, Berosus signaticollis & Platambus maculatus (all © Jonty Denton) Ireland Red List Series Editors: N. Kingston & F. Marnell © National Parks and Wildlife Service 2009 ISSN 2009‐2016 Red list of Irish Water beetles 2009 ____________________________ CONTENTS ACKNOWLEDGEMENTS .................................................................................................................................... 1 EXECUTIVE SUMMARY...................................................................................................................................... 2 INTRODUCTION................................................................................................................................................ 3 NOMENCLATURE AND THE IRISH CHECKLIST................................................................................................ 3 COVERAGE ....................................................................................................................................................... -

Helminth Communities in the Northern Spring Peeper, Pseudacris C. Crucifer Wied, and the Wood Frog, Rana Sylvatica Le Conte, from Southeastern Wisconsin
J. Helminthol. Soc. Wash. 63(2), 1996, pp. 211-214 Helminth Communities in the Northern Spring Peeper, Pseudacris c. crucifer Wied, and the Wood Frog, Rana sylvatica Le Conte, from Southeastern Wisconsin H. RANDALL YODER AND JAMES R. COGGINS Department of Biological Sciences, University of Wisconsin-Milwaukee, Milwaukee, Wisconsin 53201 ABSTRACT: Fifteen spring peepers and 20 wood frogs were collected in April 1993 from 2 temporary ponds in southeastern Wisconsin. Five species of adult and larval nematodes, 4 species of adult and larval trematodes, and 1 larval cestode infected wood frogs. Spring peepers harbored 1 adult and 1 larval nematode species as well as 1 adult and 5 larval trematode species. Sixteen of 20 (80%) wood frogs were infected with helminths. Mean species richness and mean helminth abundance were 2.15 and 4.90, respectively. Eight of 15 (53%) spring peepers were infected. Only 1 individual was infected by more than 1 helminth species and only 1 parasite species infected more than 1 spring peeper. Low prevalence and intensities of helminths as well as low diversity within infracommunities suggest depauperate, isolationist helminth communities in these 2 populations of anurans. This work represents new records for 5 helminth species in Wisconsin wood frogs and 3 helminth species in Wisconsin spring peepers. KEY WORDS: Rana sylvatica, Pseudacris crucifer, Oswaldocruzia pipiens, Cosmocercoides dukae, Rhabdias ranae, Glypthelmins pennsylvaniensis, Hematoloechus varioplexus, Fibricola texensis, Alaria mustelae, diplo- stomula, mesocercaria, Wisconsin. Rana sylvatica Le Conte is found from New and wet weight (g) were recorded. The external surfaces Brunswick to eastern Manitoba and south to as well as the mouth and eustachian tubes, the internal organs, including the brain, and the musculature of the Georgia and eastern Texas (Vogt, 1981). -

Hundreds of Species of Aquatic Macroinvertebrates Live in Illinois In
Illinois A B aquatic sowbug Asellus sp. Photograph © Paul P.Tinerella AAqquuaattiicc mayfly A. adult Hexagenia sp.; B. nymph Isonychia sp. MMaaccrrooiinnvveerrtteebbrraatteess Photographs © Michael R. Jeffords northern clearwater crayfish Orconectes propinquus Photograph © Michael R. Jeffords ruby spot damselfly Hetaerina americana Photograph © Michael R. Jeffords aquatic snail Pleurocera acutum Photograph © Jochen Gerber,The Field Museum of Natural History predaceous diving beetle Dytiscus circumcinctus Photograph © Paul P.Tinerella monkeyface mussel Quadrula metanevra common skimmer dragonfly - nymph Libellula sp. Photograph © Kevin S. Cummings Photograph © Paul P.Tinerella water scavenger beetle Hydrochara sp. Photograph © Steve J.Taylor devil crayfish Cambarus diogenes A B Photograph © ChristopherTaylor dobsonfly Corydalus sp. A. larva; B. adult Photographs © Michael R. Jeffords common darner dragonfly - nymph Aeshna sp. Photograph © Paul P.Tinerella giant water bug Belostoma lutarium Photograph © Paul P.Tinerella aquatic worm Slavina appendiculata Photograph © Mark J. Wetzel water boatman Trichocorixa calva Photograph © Paul P.Tinerella aquatic mite Order Prostigmata Photograph © Michael R. Jeffords backswimmer Notonecta irrorata Photograph © Paul P.Tinerella leech - adult and young Class Hirudinea pygmy backswimmer Neoplea striola mosquito - larva Toxorhynchites sp. fishing spider Dolomedes sp. Photograph © William N. Roston Photograph © Paul P.Tinerella Photograph © Michael R. Jeffords Photograph © Paul P.Tinerella Species List Species are not shown in proportion to actual size. undreds of species of aquatic macroinvertebrates live in Illinois in a Kingdom Animalia Hvariety of habitats. Some of the habitats have flowing water while Phylum Annelida Class Clitellata Family Naididae aquatic worm Slavina appendiculata This poster was made possible by: others contain still water. In order to survive in water, these organisms Class Hirudinea leech must be able to breathe, find food, protect themselves, move and reproduce. -

1 Project T-107-D-1: Demography, Community Dynamics, and Health of Reintroduced Wood Frog Populations and Resident Amphibian
Project T-107-D-1: Demography, community dynamics, and health of reintroduced wood frog populations and resident amphibian communities in restored ephemeral wetlands and oak woodlands in Lake County, IL. State Wildlife Grant Final Performance Report February 28, 2016-December 20, 2018 Submitted by Rachel Santymire [email protected] Lincoln Park Zoo, Department of Conservation & Science, 2001 N Cannon Dr, Chicago, IL 60614 Executive Summary Several decades of habitat degradation dramatically affected a historic assemblage of amphibians in the Upper Des Plaines region of Illinois (Sacerdote 2009). Forms of degradation included implementation of agricultural drainage tile in amphibian breeding sites, proliferation of invasive shrubs (Sacerdote and King 2014), and a history of fire suppression. These activities altered forest composition which affected pond water chemistry (Sacerdote and King 2009) and resulted in a lack of oak tree recruitment in northern Illinois. In 1999-2000, the Lake County Forest Preserve District (LCFPD) implemented a major habitat restoration initiative in MacArthur Woods Forest Preserve, focused on restoring the hydrology of the site, rehydrating 100 acres of wetland and 300 acres of hydric soil (Klick 2003). Hydrologic restoration was followed by monitoring of the amphibian community to examine whether three species of extirpated amphibians, wood frogs (Lithobates sylvaticus), spotted salamanders (Ambystoma maculatum), and spring peepers (Pseudacris crucifer) would naturally recolonize the site. When natural recolonization did not occur, Dr. Sacerdote-Velat (project co-PI) and LCFPD carried out a feasibility assessment for egg mass and larval translocation followed by implementation of reintroduction of these three species. After several years of monitoring, successful reproduction of wood frogs was first observed in 2014. -
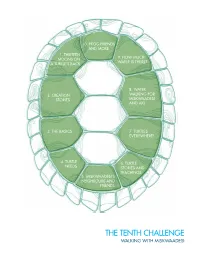
The Tenth Challenge Walking with Miskwaadesi the Tenth Challenge FROG FRIENDS and MORE
10. FROG FRIENDS AND MORE 1. THIRTEEN 9. HOW MUCH MOONS ON WATER IS THERE? A TURTLE’S BACK 8. WATER 2. CREATION WALKING FOR STORIES MISKWAADESI AND AKI 3. THE BASICS 7. TURTLES EVERYWHERE! 4. TURTLE 6. TURTLE NEEDS STORIES AND TEACHINGS 5. MISKWAADESI’S NEIGHBOURS AND FRIENDS THE TENTH CHALLENGE WALKING WITH MISKWAADESI THE TENTH CHALLENGE FROG FRIENDS AND MORE Who lives in your neighbourhood wetland? How healthy are our wet places- could Miskwaadesi live there? What role does the frog play in keeping a wetland healthy? Go out and listen for frogs and record when you hear them welcome the new season with their songs. Complete the pond study and give your wetland its very own health report card. ”…my 10th challenge is to come down to the water. It is spring and the first birds are coming back. The frogs are beginning to sing their thanksgiving song of joy. Come down to the water. Listen to the frogs - what are they saying? Record the frog clans that live in your waterways. Look at the little creatures who live in and around the water. Come down to the water and become close to the spirit of the water and the land.” Miskwaadesi’s 10th challenge. 260 EXPECTATIONS PRACTICING THE LEARNING | FOLLOWING THE FOOTSTEPS TITLE OF ACTIVITY ONTARIO CURRICULUM EXPECTATION WORKSHEET Planning and gathering 4s4, 4s10, 4s6 Getting Ready to Go equipment A Visit to the Pond 4s21, 4s15, 4s6 Field trip DEMONSTRATING THE LEARNING | MAKING OUR OWN FOOTSTEPS TITLE OF ACTIVITY ONTARIO CURRICULUM EXPECTATION WORKSHEET Field trip and survey Frog Watch 4s6, 4s19 worksheet Wetland Report Card 4s4, 4s14 Chart/table Journal Reflection 4s5, 4s15 ONE STEP MORE (individual student optional adventures in learning) 1. -

Algae and Invertebrates of a Great Basin Desert Hot Lake: a Description of the Borax Lake Ecosystem of Southeastern Oregon
Conference Proceedings. Spring-fed Wetlands: Important Scientific and Cultural Resources of the Intermountain Region, 2002. http://www.wetlands.dri.edu Algae and Invertebrates of a Great Basin Desert Hot Lake: A description of the Borax Lake ecosystem of southeastern Oregon Joseph Furnish Pacific Southwest Region 5, U.S. Department of Agriculture, Forest Service, Vallejo, CA [email protected] James McIver Pacific Northwest Research Station, U.S. Department of Agriculture, Forest Service, LaGrande, OR Mark Teiser Department of Oceanography, Oregon State University, Corvallis, OR Abstract Introduction As part of the recovery plan for the Borax Lake is a geothermally heated endangered chub Gila boraxobius (Cyprinidae), alkaline lake in southeastern Oregon. It a description of algal and invertebrate represents one of the only permanent water populations was undertaken at Borax Lake in sources in the Alvord Desert, which receives 1991 and 1992. Borax Lake, the only known less than 20 cm of rain annually (Green 1978; habitat for G. boraxobius, is a warm, alkaline Cobb et al. 1981). Borax Lake is the only known water body approximately 10 hectares in size habitat for Gila boraxobius, the Borax Lake with an average surface water temperature of chub, a cyprinid fish recognized as a new 30°C. Periphyton algae were surveyed by species in 1980. The chub was listed as scraping substrates and incubating microscope endangered under the Endangered Species Act slides in the water column. Invertebrates were in 1982 because it was believed that geothermal- collected using dip nets, pitfall traps and Ekman energy test-well drilling activities near Borax dredges. The aufwuchs community was Lake might jeopardize its habitat by altering the composed of 23 species and was dominated by flow or temperature of water in the lake. -

Fifield View PDF
St. Olaf College Local Ecology Research Papers Presence and Absence Survey of Wetland Anurans in St. Olaf College Natural Lands Cindy Fifield 2016 © Cindy Fifield, 2016 “Presence and Absence Survey of Wetland Anurans in St. Olaf College Natural Lands” by Cindy Fifield is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. Presence and Absence Survey of Wetland Anurans in St. Olaf College Natural Lands Cindy Fifield St. Olaf College 1500 St. Olaf Ave Northfield, MN 55057 Spring 2016 Introduction Anuran populations are experiencing a global population decline resulting in dramatic losses in biodiversity. Low population numbers indicate habitats have declined in quantity and quality. Currently over one third of amphibian populations are threatened globally (Hamer & Mcdonnell, 2008). In addition to global declines in amphibians, many midwestern populations show signs of stress. During the 1990’s, a significant proportion of midwestern frogs presented large numbers of limb abnormalities consistent with mutations found in vertebrates exposed to exogenous retinoids during development (Gardiner & Hoppe 1999). Researchers also suspected parasitism and UV radiation as potential influences, however no survey reached a clear conclusion (Reister et. al 1998). Given the elusiveness of the cause and the frequency of malformation, there is growing concern for the status of Midwestern anurans populations. Besides these developmental disturbances, anuran populations also face disturbance through habitat loss from urbanization and agriculture. This is problematic not only because anurans are important in maintaining biodiversity, but also because they are an indicator of environmental integrity. Anuran surveys are often used as indicators of ecosystem disturbance because they are a sensitive species (Hager 1998). -

Amphibian Background
The Toledo Zoo/ThinkingWorks Teacher Overview for the Amphibian Lessons Ó2003 Teacher Overview: Amphibians Amphibians have many traits that are unique to this particular class of animals. Below is a list of general amphibian traits to help you and your students complete the ThinkingWorks lesson. The class Amphibia is divided into three groups or orders, each with their own set of features. The orders are frogs and toads, salamanders and caecilians. We have included a list of the different amphibians found at The Toledo Zoo by order and where you can find them on exhibit. Note that animals move constantly in and out of the Zoo. Please call the Zoo for a current list of amphibians that are on exhibit and their locations. Wild toad tadpoles and adults can also be observed on Zoo grounds in the formal garden area near the Conservatory. Look near the pool in the butterfly garden. General Amphibian Traits q The life cycle of an amphibian (the name means “two-lived”) begins as an egg. Tadpoles or larvae (singular is larva) hatch from the egg. Tadpoles are an immature stage. The tadpoles then mature into adults. This process is called metamorphosis. q Females deposit eggs in water where they are fertilized externally by males (see diagram). q Eggs hatch into tadpoles that are aquatic (live in water), breathe through gills instead of lungs, have a tail, no eyelids, ears and, initially, no legs. Adult Male Tadpole (Larva) Adult Female Eggs q Adults of most amphibians have four legs, lungs, eyelids, tear glands and ears. q Besides lungs, most adult amphibians can exchange gases through the skin and the membranes lining the mouth. -

Insect Egg Size and Shape Evolve with Ecology but Not Developmental Rate Samuel H
ARTICLE https://doi.org/10.1038/s41586-019-1302-4 Insect egg size and shape evolve with ecology but not developmental rate Samuel H. Church1,4*, Seth Donoughe1,3,4, Bruno A. S. de Medeiros1 & Cassandra G. Extavour1,2* Over the course of evolution, organism size has diversified markedly. Changes in size are thought to have occurred because of developmental, morphological and/or ecological pressures. To perform phylogenetic tests of the potential effects of these pressures, here we generated a dataset of more than ten thousand descriptions of insect eggs, and combined these with genetic and life-history datasets. We show that, across eight orders of magnitude of variation in egg volume, the relationship between size and shape itself evolves, such that previously predicted global patterns of scaling do not adequately explain the diversity in egg shapes. We show that egg size is not correlated with developmental rate and that, for many insects, egg size is not correlated with adult body size. Instead, we find that the evolution of parasitoidism and aquatic oviposition help to explain the diversification in the size and shape of insect eggs. Our study suggests that where eggs are laid, rather than universal allometric constants, underlies the evolution of insect egg size and shape. Size is a fundamental factor in many biological processes. The size of an 526 families and every currently described extant hexapod order24 organism may affect interactions both with other organisms and with (Fig. 1a and Supplementary Fig. 1). We combined this dataset with the environment1,2, it scales with features of morphology and physi- backbone hexapod phylogenies25,26 that we enriched to include taxa ology3, and larger animals often have higher fitness4. -

Spring 2021 Spring Creative Ways Ways Creative
ACADIA 43 Cottage Street, PO Box 45 Bar Harbor, ME 04609 SPRING 2021 Volume 26 No. 2 SPRING 2021 Volume The Friends of Acadia Journal SPRING 2021 MISSION Friends of Acadia preserves, protects, and promotes stewardship of the outstanding natural beauty, ecological vitality, and distinctive cultural resources of Acadia National Park and surrounding communities for the inspiration and enjoyment of current and future generations. VISITORS enjoy a game of cribbage while watching the sunset from Beech Mountain. ACADIA OPENS RESERVATION CREATIVE WAYS FOR THE 2021 SEASON SYSTEM TO SUPPORT ACADIA TO IMPROVE VISITOR EXPERIENCE ASHLEY L. CONTI/FOA friendsofacadia.org | 43 Cottage Street | PO Box 45 | Bar Harbor, ME | 04609 | 207-288-3340 | 800 - 625- 0321 PURCHASE YOUR PARK PASS! Whether walking, bicycling, riding the Island Explorer, or driving through the park, we all must obtain a park pass. Eighty percent of all fees paid in Acadia National Park stay in Acadia, to be used for projects that directly benefit park visitors and resources. BUY A PASS ONLINE AND PRINT Acadia National Park passes are available online: before you arrive at the park. This www.recreation.gov/sitepass/74271 allows you to drive directly to a Annual park passes are also available at trailhead/parking area & display certain Acadia-area town offices and local your pass from your vehicle. chambers of commerce. Visit www.nps.gov/acad/planyourvisit/fees.htm IN THIS ISSUE 10 8 12 20 18 FEATURES 6 REMEMBERING DIANNA EMORY Our Friend, Conservationist, and Defender of Acadia By David -

Maine Breeding Bird Atlas
Maine Breeding Bird Atlas Atlasing Crepuscular and Nocturnal Birds in Your Block March 2019, version 2.1 A Project of the Maine Department of Inland Fisheries and Wildlife Maine Breeding Bird Atlas - Crepuscular and Nocturnal Observations Guidance Every evening, as the sun sets and the moon rises, the cast of active birds shifts. As diurnal (day- time) birds such as woodpeckers, waterfowl, shorebirds, hawks, eagles, and most songbirds go silent and settle in for the night, crepuscular (twilight) and nocturnal (nighttime) birds such as nightjars, owls, and handful of others emerge. While you are likely to spend the majority of your time collecting breeding bird observations in your block during the day, we encourage you to go out and collect observations during these time periods as well. These special surveys will help fill an important information gap in our atlas efforts. It is just as important to document where birds are as it is to document where birds are not - so all surveys are very helpful, even if you don’t find any crepuscular or nocturnal species When to survey: Crepuscular: Survey anytime during the twilight hours from 45 minutes before to 45 minutes af- ter sunset. Birds to look and listen for during this window include Common Nighthawk, Common Loon (in blocks near lakes), Chimney Swifts, American Woodcock, Wilson’s Snipe, and thrushes such as Veery, Swainson’s Thrush, Hermit Thrush, Wood Thrush, and American Robin (see spe- cies accounts at the end of this document for more information). Nocturnal: Survey anytime after dark from 45 minutes after sunset to approximately 45 minutes before sunrise. -

Diving Beetle Offspring Oviposited in Amphibian Spawn Prey on the Tadpoles Upon Hatching
bioRxiv preprint doi: https://doi.org/10.1101/666008; this version posted June 10, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 Diving beetle offspring oviposited in amphibian spawn prey on the tadpoles upon hatching John Gould1*, Jose W. Valdez2, Simon Clulow1,3, John Clulow1 1School of Environmental and Life Sciences, University of Newcastle, Callaghan, 2308 NSW, Australia 2 Department of Bioscience - Biodiversity and Conservation, Aarhus University, 8410 Rønde, Denmark 3Department of Biological Sciences, Macquarie University, Sydney, 2019 NSW, Australia * [email protected] Abstract In highly ephemeral freshwater habitats, predatory vertebrates are typically unable to become established, leaving an open niche often filled by macroinvertebrate predators. However, these predators are faced with the challenge of finding sufficient food sources as the rapid rate of desiccation prevents the establishment of extended food chains and limits the number of prey species present. It could therefore be advantageous for adults to oviposit their offspring in the presence of future prey within sites of extreme ephemerality. We report the first case of adult diving beetles ovipositing their eggs within spawn of the sandpaper frog, Lechriodus fletcheri. This behaviour was found among several pools used by L. fletcheri for reproduction. Beetle eggs oviposited in frog spawn were found to hatch within 24 hours of the surrounding L. fletcheri eggs, with the larvae becoming voracious consumers of the hatched tadpoles.