Sample Chapter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Calciphylaxis with Normal Renal and Parathyroid Function Not As Rare As Previously Believed
OBSERVATION Calciphylaxis With Normal Renal and Parathyroid Function Not as Rare as Previously Believed Andrew H. Kalajian, MD; Paula S. Malhotra, MD; Jeffrey P. Callen, MD; Lynn P. Parker, MD Background: Calciphylaxis is a life-threatening form of previously reported cases of nontraditional calciphylaxis metastatic calcification-induced microvascular occlusion identified the following patient characteristics that high- syndrome. Although traditionally observed in patients with light clinical situations potentially predisposing to calci- end-stage renal disease and/or hyperparathyroidism, the phylaxis: hypoalbuminemia, malignant neoplasm, sys- development of calciphylaxis in “nontraditional” pa- temic corticosteroid use, anticoagulation with warfarin tients having both normal renal and parathyroid func- sodium or phenprocoumon, chemotherapy, systemic in- tion has been reported. However, to date there has been flammation, hepatic cirrhosis, protein C or S deficiency, no collective analysis identifying common patient char- obesity, rapid weight loss, and infection. acteristics potentially predisposing to the development of calciphylaxis in nontraditional patients. Conclusions: Calciphylaxis is becoming increasingly common in patients with normal renal and parathyroid Observations: A 58-year-old woman with endometrial function. The observations from this study may assist der- carcinoma developed extensive calciphylaxis despite the matologists in the rapid diagnosis and prompt initiation presence of normal renal and parathyroid function. -

Soft Tissue Calcification and Ossification
Soft Tissue Calcification and Ossification Soft-tissue Calcification Metastatic Calcification =deposit of calcium salts in previously normal tissue (1) as a result of elevation of Ca x P product above 60-70 (2) with normal Ca x P product after renal transplant Location:lung (alveolar septa, bronchial wall, vessel wall), kidney, gastric mucosa, heart, peripheral vessels Cause: (a)Skeletal deossification 1.1° HPT 2.Ectopic HPT production (lung / kidney tumor) 3.Renal osteodystrophy + 2° HPT 4.Hypoparathyroidism (b)Massive bone destruction 1.Widespread bone metastases 2.Plasma cell myeloma 3.Leukemia Dystrophic Calcification (c)Increased intestinal absorption =in presence of normal serum Ca + P levels secondary to local electrolyte / enzyme alterations in areas of tissue injury 1.Hypervitaminosis D Cause: 2.Milk-alkali syndrome (a)Metabolic disorder without hypercalcemia 3.Excess ingestion / IV administration of calcium salts 1.Renal osteodystrophy with 2° HPT 4.Prolonged immobilization 2.Hypoparathyroidism 5.Sarcoidosis 3.Pseudohypoparathyroidism (d)Idiopathic hypercalcemia 4.Pseudopseudohypoparathyroidism 5.Gout 6.Pseudogout = chondrocalcinosis 7.Ochronosis = alkaptonuria 8.Diabetes mellitus (b) Connective tissue disorder 1.Scleroderma 2.Dermatomyositis 3.Systemic lupus erythematosus (c)Trauma 1.Neuropathic calcifications 2.Frostbite 3.Myositis ossificans progressiva 4.Calcific tendinitis / bursitis (d)Infestation 1.Cysticercosis Generalized Calcinosis 2.Dracunculosis (guinea worm) (a)Collagen vascular disorders 3.Loiasis 1.Scleroderma -

The Association Between Hypocalcemia and Outcome in COVID-19 Patients: a Retrospective Study
The Association Between Hypocalcemia and Outcome in COVID-19 Patients: a Retrospective Study Bhagwan Singh Patidar All India Institute of Medical Sciences Tapasyapreeti Mukhopadhayay All India Institute of Medical Sciences Arulselvi Subramanian ( [email protected] ) All India Institute of Medical Sciences https://orcid.org/0000-0001-7797-6683 Riicha Aggarwal All India Institute of Medical Sciences Kapil Dev Soni All India Institute of Medical Sciences Neeraj Nischal All India Institute of Medical Sciences Debasis Sahoo All India Institute of Medical Sciences Surbhi Surbhi All India Institute of Medical Sciences Ravindra Mohan Pandey All India Institute of Medical Sciences Naveet Wig All India Institute of Medical Sciences Rajesh Malhotra All India Institute of Medical Sciences Anjan Trikha All India Institute of Medical Sciences Research Article Keywords: Calcium, Coronavirus, Laboratory parameters, Mortality, NLR, Pandemic Posted Date: March 16th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-302159/v1 Page 1/14 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 2/14 Abstract Background: Calcium has been shown to have a vital role in the pathophysiology of SARS-CoV and MERS-CoV diseases but less is known about hypocalcemia in COVID-19 patients and its association with the disease severity and the nal outcome. Therefore, this study was conducted with an aim to assess the clinical features in the COVID-19 patients having hypocalcemia and to observe its impact on COVID- 19 disease severity and nal outcome. Method: In this retrospective study, consecutive COVID-19 patients of all age groups were enrolled. -

Epileptic Seizure, As the First Symptom of Hypoparathyroidism in Children, Does Not Require Antiepileptic Drugs
Childs Nerv Syst DOI 10.1007/s00381-016-3264-2 ORIGINAL PAPER Epileptic seizure, as the first symptom of hypoparathyroidism in children, does not require antiepileptic drugs Meng-Jia Liu1 & Jiu-Wei Li2 & Xiu-Yu Shi1 & Lin-Yan Hu1 & Li-Ping Zou1,3 Received: 28 May 2016 /Accepted: 3 October 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Introduction Objective Patients with hypoparathyroidism exhibit metabol- ic disorders (hypocalcemia) and brain structural abnormalities Epileptic seizure occurs when a burst of electrical impulses in (brain calcifications). Currently, studies have determined the brain exceeds the normal limits. Its manifestation can vary whether antiepileptic drug (AED) treatment is required for from uncontrolled jerking movement (tonic–clonic seizure) to epileptic seizures in children with hypoparathyroidism. momentary loss of awareness (absence seizure). These im- Method This study aims to evaluate the data of two medical pulses spread to adjacent areas in the brain and create an un- centers in Beijing based on the diagnosis of epileptic seizures controlled storm of electrical activity. Brain diseases character- as the first symptom of hypoparathyroidism in children. ized by enduring predisposition to generate epileptic seizures Result A total of 42 patients were included and assigned into are collectively called epilepsy. According to pathogenesis, ep- AED and non-AED treatment groups in a 1:2 matched case– ilepsy can be classified into six categories: metabolic, structural, control study. Results show that the seizure outcome after inherited, immunologic, inflammatory, and idiopathic. 1 year of AED treatment is not significantly different from Hypoparathyroidism is an endocrine disease that results that of the control. -

Electrolyte Disorders in Cancer Patients: a Systematic Review
Berardi et al. J Cancer Metastasis Treat 2019;5:79 Journal of Cancer DOI: 10.20517/2394-4722.2019.008 Metastasis and Treatment Review Open Access Electrolyte disorders in cancer patients: a systematic review Rossana Berardi, Mariangela Torniai, Edoardo Lenci, Federica Pecci, Francesca Morgese, Silvia Rinaldi Clinica Oncologica, Università Politecnica delle Marche, Azienda Ospedaliero-Universitaria Ospedali Riuniti Umberto I - GM Lancisi - G Salesi, Ancona 60126, Italy. Correspondence to: Prof. Rossana Berardi, Clinica Oncologica, Università Politecnica delle Marche, Azienda Ospedaliero- Universitaria Ospedali Riuniti di Ancona, Via Conca 71, Ancona 60126, Italy. E-mail: [email protected] How to cite this article: Berardi R, Torniai M, Lenci E, Pecci F, Morgese F, Rinaldi S. Electrolyte disorders in cancer patients: a systematic review. J Cancer Metastasis Treat 2019;5:79. http://dx.doi.org/10.20517/2394-4722.2019.008 Received: 26 Apr 2019 First Decision: 26 Jul 2019 Revised: 20 Nov 2019 Accepted: 20 Nov 2019 Published: 9 Dec 2019 Science Editor: Stephen J. Ralph Copy Editor: Jing-Wen Zhang Production Editor: Jing Yu Abstract Electrolyte disorders are very common complications in cancer patients. They might be associated to a worsening outcome, influencing quality of life, possibility to receive anticancer drugs, and conditioning survival. In fact, they might provoke important morbidity, with dysfunction of multiple organs and rarely causing life-threatening conditions. Moreover, recent studies showed that they might worsen cancer patients’ outcome, while a prompt correction seems to have a positive impact. Furthermore, there is evidence of a correlation between electrolyte alterations and poorer performance status, delays in therapy commencement and continuation, and negative treatment outcomes. -

Clinical Physiology Aspects of Chloremia in Fluid Therapy: a Systematic Review David Astapenko1,2* , Pavel Navratil2,3, Jiri Pouska4,5 and Vladimir Cerny1,2,6,7,8,9
Astapenko et al. Perioperative Medicine (2020) 9:40 https://doi.org/10.1186/s13741-020-00171-3 REVIEW Open Access Clinical physiology aspects of chloremia in fluid therapy: a systematic review David Astapenko1,2* , Pavel Navratil2,3, Jiri Pouska4,5 and Vladimir Cerny1,2,6,7,8,9 Abstract Background: This systematic review discusses a clinical physiology aspect of chloride in fluid therapy. Crystalloid solutions are one of the most widely used remedies. While generally used in medicine for almost 190 years, studies focused largely on their safety have only been published since the new millennium. The most widely used solution, normal saline, is most often referred to in this context. Its excessive administration results in hyperchloremic metabolic acidosis with other consequences, including higher mortality rates. Methods: Original papers and review articles eligible for developing the present paper were identified by searching online in the electronic MEDLINE database. The keywords searched for included hyperchloremia, hypochloremia, and compound words containing the word “chloride,” infusion therapy, metabolic acidosis, renal failure, and review. Results: A total of 21,758 papers published before 31 May 2020 were identified; of this number, 630 duplicates were removed from the list. Upon excluding articles based on their title or abstract, 1850 papers were screened, of which 63 full-text articles were assessed. Conclusions: According to the latest medical concepts, dyschloremia (both hyperchloremia and hypochloremia) represents a factor indisputably having a negative effect on selected variables of clinical outcome. As infusion therapy can significantly impact chloride homeostasis of the body, the choice of infusion solutions should always take into account the potentially adverse impact of chloride content on chloremia and organ function. -

THE EFFECT of POTASSIUM CHLORIDE on HYPONATREMIA1 by JOHN H
THE EFFECT OF POTASSIUM CHLORIDE ON HYPONATREMIA1 By JOHN H. LARAGH2 (From the Department of Medicine, College of Physicians and Surgeons, Columbia University; and the Presbyterian Hospital in the City of New York) (Submitted for publication August 31, 1953; accepted February 10, 1954) In cardiac edema as well as in various other tration might favorably influence disturbances in states characterized by excessive retention of fluid sodium metabolism manifested by pathologic dis- there is observed frequently an abnormally low con- tribution of sodium and potassium within the body. centration of sodium in the serum and extracellular Two recent studies have served to emphasize this fluid (1, 2). Rigorous sodium restriction, mer- important relationship between sodium and potas- curial diuretics, and cation exchange resins may sium. In vivo, with potassium depletion there is exaggerate or produce this tendency to hypo- a movement of sodium ions into cells and an associ- tonicity. Sodium administration often enhances ated extracellular alkalosis. This intracellular so- accumulation of fluid, without increasing its to- dium can then be mobilized by potassium adminis- nicity, and hypertonic sodium chloride, though tration (11). In vitro it has been shown that po- effective at times, may be neither beneficial nor tassium transport is dependent upon energy of safe (3). aerobic oxidation. If the cell is injured or meta- Because of the shortcomings of these various bolically inhibited potassium fails to accumulate therapies and because hyponatremia per se may and is replaced by an influx of sodium and water play a role in the production of adverse symptoms, (12). it seemed desirable to search for another diuretic Accordingly, potassium chloride (KCl) was agent which might promote the loss of excessive given to an edematous, hyponatremic cardiac body water without aggravating disturbances in patient with the hope of effecting water diuresis. -
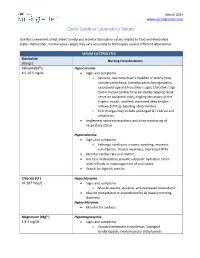
Quick Guide to Laboratory Values
March 2021 www.nursingcenter.com Quick Guide to Laboratory Values Use this convenient cheat-sheet to help you monitor laboratory values related to fluid and electrolyte status. Remember, normal value ranges may vary according to techniques used in different laboratories. SERUM ELECTROLYTES Electrolyte Nursing Considerations (Range) Calcium (Ca2+) Hypocalcemia 8.5-10.5 mg/dL • Signs and symptoms o Seizures, neuromuscular irritability or tetany (may include paresthesia, bronchospasm, laryngospasm, carpopedal spasm [Trousseau’s sign], Chvostek’s sign [facial muscle contractions elicited by tapping facial nerve on ipsilateral side], tingling sensations of the fingers, mouth, and feet, increased deep tendon reflexes [DTRs]), bleeding abnormalities o ECG changes may include prolonged QT interval and arrythmias. • Implement seizure precautions and close monitoring of respiratory status. Hypercalcemia • Signs and symptoms o Lethargy, confusion, nausea, vomiting, anorexia, constipation, muscle weakness, depressed DTRs • Monitor cardiac rate and rhythm. • Increase mobilization, provide adequate hydration either with IV fluids or encouragement of oral intake. • Watch for digitalis toxicity. Chloride (Cl-) Hypochloremia 97-107 mEq/L • Signs and symptoms o Muscle spasms, alkalosis, and depressed respirations • May be precipitated or exacerbated by GI losses (vomiting, diarrhea). Hyperchloremia • Monitor for acidosis. Magnesium (Mg2+) Hypomagnesemia 1.8-3 mg/dL • Signs and symptoms o Cardiac/ventricular arrhythmias, laryngeal stridor/spasm, neuromuscular -
Fluid and Electrolyte Balance
Fluid and Electrolyte Balance Done By: Faisal S. AlGhamdi Abdullah Almousa Total Body Fluids and fluids compartment: 60% in male of Total body weight 55% in female 2/3 (65%) of TBW is intracellular (ICF) 1/3 (35%) extracellular water – 25 % interstitial fluid (ISF) – 5 - 7 % in plasma (IVF intravascular fluid) – 1- 2 % in transcellular fluids – CSF, intraocular fluids, serous membranes, and in GI, respiratory and urinary tracts (third space) • Fluid compartments are separated by membranes that are freely permeable to water. • Movement of fluids due to: – Hydrostatic pressure (Fluid) – Osmotic/Oncotic pressure (tissue) In Hydrostatic pressure: As the pressure increase as the movement of fluid outside increase In Osmotic pressure: As the pressure increase as the absorption of fluid increase. Fluid balance: • Neutral balance: input = output • Positive balance: input > output • Negative balance: input < output (+ve lead to edema, and -ve lead to dehydration) Daily input should = Daily output Most of water intake in Beverages Most of water output in Urine Electrolytes: Cations – positively charged ions . Na+, K+ , Ca++, H+ Anions – negatively charged ions - - 3- . Cl , HCO3 , PO4 Intracellular fluid space: • 40% of body weight • Largest proportion is in skeletal muscle • Larger percentage of water is Intracellular in males (large muscle mass) • Cations = Potassium & Magnesium • Anions = Phosphates and Proteins Extracellular fluid space: • 20% of body weight • Interstitial 15%, Plasma 5% • Cations = Sodium • Anions = Chloride and Bicarbonate Homeostasis: • Maintained by Ion transport, Water movement and Kidney function. • Tonicity Isotonic, Hypertonic and Hypotonic (the difference btw tonicity and osmolarity that tonicity is concentration of solutions in relation to adjacent compartments *like concentration of plasma compared to interstitial space*, but osmolarity take the compartment on it’s own) Movement of body fluids: “ Where Na goes, H2O follows” Diffusion – movement of particles down a concentration gradient. -

DKA Protocol - Insulin Deficiency - Pregnancy
Diabetic Ketoacidosis in Pregnancy Diagnosis of DKA: Initial STAT labs include • CBC with diff • Serum electrolytes • BUN • Creatinine • Glucose • Arterial blood gases • Bicarbonate • Urinalysis • Lactate • Serum ketones • Calculation of the Anion Gap serum anion gap = serum sodium – (serum chloride + bicarbonate) • Electrocardiogram Treatment Protocol for Diabetic Ketoacidosis Reviewed 5/2/2017 1 Updated 05/02/17 DKA/HHS Pathway Phase 1 (Adult) DKA Diagnostic Criteria: Blood glucose >250 mg/dl *PREGNANCY Arterial pH <7.3 Utilize OB DKA order set Phase 1 Bicarbonate ≤18 mEq/l When glucose reaches 200mg/dL, Initiate OB Anion Gap Acidosis DKA Phase 2 Moderate ketonuria or ketonemia Glucose goals 100 -150mg/dL OB DKA Phase 2 1. Start IV fluids (1 L of 0.9% NaCl per hr initially) Look for the Cause 2. If serum K+ is <3.3 mEq/L hold insulin - Infection/Inflammation (PNA, UTI, Give 40 mEq/h until K ≥ 3.3 mEq/L pancreatitis, cholecystitis) 3. Initiate DKA Order Set Phase I ( *In PREGNANCY utilize OB DKA - Ischemia/Infarction (myocardial, cerebral, gut) order set ) - Intoxication (EtOH, drugs) 4. Start insulin 0.14 units/kg/hr IV infusion (calculate dose) - Iatrogenic (drugs, lack of insulin) RN will titrate per DKA protocol - Insulin deficiency - Pregnancy IVF Insulin Potassium Bicarbonate + Determine hydration status Initiate and If initial serum K is Assess need for bicarbonate continue insulin gtt <3.3 mEq/L, hold until serum insulin and give 40 + glucose reaches mEq K per h (2/3 250 mg/dl. KCL and 1/3 KP0 ) Hypovolemic Mild Cardiogenic 4 pH <6.9 pH >7.0 shock hypotension shock RN will titrate per until K ≥ 3.3 mEq/L protocol to achieve target. -

Acid-Base Physiology & Anesthesia
ACID-BASE PHYSIOLOGY & ANESTHESIA Lyon Lee DVM PhD DACVA Introductions • Abnormal acid-base changes are a result of a disease process. They are not the disease. • Abnormal acid base disorder predicts the outcome of the case but often is not a direct cause of the mortality, but rather is an epiphenomenon. • Disorders of acid base balance result from disorders of primary regulating organs (lungs or kidneys etc), exogenous drugs or fluids that change the ability to maintain normal acid base balance. • An acid is a hydrogen ion or proton donor, and a substance which causes a rise in H+ concentration on being added to water. • A base is a hydrogen ion or proton acceptor, and a substance which causes a rise in OH- concentration when added to water. • Strength of acids or bases refers to their ability to donate and accept H+ ions respectively. • When hydrochloric acid is dissolved in water all or almost all of the H in the acid is released as H+. • When lactic acid is dissolved in water a considerable quantity remains as lactic acid molecules. • Lactic acid is, therefore, said to be a weaker acid than hydrochloric acid, but the lactate ion possess a stronger conjugate base than hydrochlorate. • The stronger the acid, the weaker its conjugate base, that is, the less ability of the base to accept H+, therefore termed, ‘strong acid’ • Carbonic acid ionizes less than lactic acid and so is weaker than lactic acid, therefore termed, ‘weak acid’. • Thus lactic acid might be referred to as weak when considered in relation to hydrochloric acid but strong when compared to carbonic acid. -

Hyponatremia in Hepatic Cirrhosis Following Paracentesis
HYPONATREMIA IN HEPATIC CIRRHOSIS FOLLOWING PARACENTESIS William P. Nelson III, … , Jack D. Rosenbaum, Maurice B. Strauss J Clin Invest. 1951;30(7):738-744. https://doi.org/10.1172/JCI102487. Research Article Find the latest version: https://jci.me/102487/pdf HYPONATREMIA IN HEPATIC CIRRHOSIS FOLLOWING PARACENTESIS 1 By WILLIAM P. NELSON, III, JACK D. ROSENBAUM, AND MAURICE B. STRAUSS (From the Medical Service, Cushing Veterans Administration Hospital, Framingham, Mass.) (Submitted for publication January 15, 1951; accepted April 23, 1951) The retention of water without a physiologically modification of the Folin procedure (14); non-protein equivalent amount of sodium following abdominal nitrogen by micro-Kjeldahl with Nesslerization (15); and sodium and potassium by means of the Barclay internal paracentesis has been studied in two patients with standard flame photometer. Except where otherwise advanced cirrhosis of the liver. In each there de- noted, urine was collected over 24 hour periods and was veloped manifestations considered characteristic analyzed for chloride by the Volhard-Harvey method of sodium deficit, although there was no change in (16), for total nitrogen by the micro-Kjeldahl procedure the total body sodium at the time these appeared. (11), and for creatinine, sodium, and potassium by the methods employed for serum. Change in total body water Such retention of water in excess of salt, regularly (liters) was taken to equal change in weight (kilograms). observed when large external losses of salt and water are replaced with water alone (1-3), has CASE REPORTS AND RESULTS been noted in certain cases of heart failure and chronic renal disease (4-6) as well as in decom- Case I.