Kabog Millet” As an Alternative to Rice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phytochemical Profiles of Black, Red, Brown, and White Rice from The
Article pubs.acs.org/JAFC Phytochemical Profiles of Black, Red, Brown, and White Rice from the Camargue Region of France Gema Pereira-Caro,† Gerard Cros,‡ Takao Yokota,¶ and Alan Crozier*,† † Joseph Black Building, School of Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G12 8QQ, United Kingdom ‡ Laboratoire de Pharmacologie, CNRS UMR 5247 and UniversitéMontpellier 1 and 2, Institut des Biomoleculeś Max Mousseron, Facultéde Pharmacie, 15 avenue Charles Flahault, Montpellier 34093 cedex 05, France ¶ Department of Biosciences, Teikyo University, Utsunomiya 320-8551, Japan ABSTRACT: Secondary metabolites in black, red, brown, and white rice grown in the Camargue region of France were investigated using HPLC-PDA-MS2. The main compounds in black rice were anthocyanins (3.5 mg/g), with cyanidin 3-O- glucoside and peonidin 3-O-glucoside predominating, followed by flavones and flavonols (0.5 mg/g) and flavan-3-ols (0.3 mg/g), which comprised monomeric and oligomeric constituents. Significant quantities of γ-oryzanols, including 24-methylenecy- cloartenol, campesterol, cycloartenol, and β-sitosterol ferulates, were also detected along with lower levels of carotenoids (6.5 μg/ g). Red rice was characterized by a high amount of oligomeric procyanidins (0.2 mg/g), which accounted >60% of secondary metabolite content with carotenoids and γ-oryzanol comprising 26.7%, whereas flavones, flavonols and anthocyanins were <9%. Brown and white rice contained lower quantities of phytochemicals, in the form of flavones/flavonols (21−24 μg/g) and γ- oryzanol (12.3−8.2 μg/g), together with trace levels of the carotenoids lutein and zeaxanthin. Neither anthocyanins nor procyanidins were detected in brown and white rice. -

Iaea Tecdoc 1847
IAEA-TECDOC-1847 IAEA-TECDOC-1847 IAEA TECDOC SERIES Rice Production Guidelines: Best Farm Management Practices and the Role of Isotopic Techniques Management Practices and the Role of Isotopic Best Farm Rice Production Guidelines: IAEA-TECDOC-1847 Rice Production Guidelines: Best Farm Management Practices and the Role of Isotopic Techniques International Atomic Energy Agency Vienna ISBN 978–92–0–103418–2 ISSN 1011–4289 @ RICE PRODUCTION GUIDELINES: BEST FARM MANAGEMENT PRACTICES AND THE ROLE OF ISOTOPIC TECHNIQUES The following States are Members of the International Atomic Energy Agency: AFGHANISTAN GHANA PANAMA ALBANIA GREECE PAPUA NEW GUINEA ALGERIA GRENADA PARAGUAY ANGOLA GUATEMALA PERU ANTIGUA AND BARBUDA GUYANA PHILIPPINES ARGENTINA HAITI POLAND ARMENIA HOLY SEE PORTUGAL AUSTRALIA HONDURAS QATAR AUSTRIA HUNGARY REPUBLIC OF MOLDOVA AZERBAIJAN ICELAND ROMANIA BAHAMAS INDIA RUSSIAN FEDERATION BAHRAIN INDONESIA BANGLADESH IRAN, ISLAMIC REPUBLIC OF RWANDA BARBADOS IRAQ SAINT VINCENT AND BELARUS IRELAND THE GRENADINES BELGIUM ISRAEL SAN MARINO BELIZE ITALY SAUDI ARABIA BENIN JAMAICA SENEGAL BOLIVIA, PLURINATIONAL JAPAN SERBIA STATE OF JORDAN SEYCHELLES BOSNIA AND HERZEGOVINA KAZAKHSTAN SIERRA LEONE BOTSWANA KENYA SINGAPORE BRAZIL KOREA, REPUBLIC OF SLOVAKIA BRUNEI DARUSSALAM KUWAIT SLOVENIA BULGARIA KYRGYZSTAN SOUTH AFRICA BURKINA FASO LAO PEOPLE’S DEMOCRATIC SPAIN BURUNDI REPUBLIC SRI LANKA CAMBODIA LATVIA SUDAN CAMEROON LEBANON SWAZILAND CANADA LESOTHO SWEDEN CENTRAL AFRICAN LIBERIA SWITZERLAND REPUBLIC LIBYA CHAD LIECHTENSTEIN SYRIAN -

Research Article Effect of Microwave Cooking on Quality of Riceberry Rice (Oryza Sativa L.)
Hindawi Journal of Food Quality Volume 2020, Article ID 4350274, 9 pages https://doi.org/10.1155/2020/4350274 Research Article Effect of Microwave Cooking on Quality of Riceberry Rice (Oryza sativa L.) Lyda Chin, Nantawan Therdthai , and Wannasawat Ratphitagsanti Department of Product Development, Faculty of Agro-Industry, Kasetsart University, Bangkok 10900, #ailand Correspondence should be addressed to Nantawan erdthai; [email protected] Received 9 October 2019; Revised 8 August 2020; Accepted 13 August 2020; Published 28 August 2020 Academic Editor: Mar´ıa B. Pe´rez-Gago Copyright © 2020 Lyda Chin et al. is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Microwaves have been applied for cooking, warming, and thawing food for many years. Microwave heating differs from conventional heating and may cause variation in the food quality. is study determined the quality of Riceberry rice (Oryza sativa L.) after microwave cooking using various rice-to-water ratios at three power levels (360, 600, and 900 W). e texture of all microwave-cooked samples was in the range 162.35 ± 5.86 to 180.11 ± 7.17 N and was comparable to the conventionally cooked rice (162.03 N). e total phenolic content (TPC) and the antioxidant activity of the microwave-cooked rice were higher than those of the conventional-cooked rice. Microwave cooking appeared to keep the TPC in the range 241.15–246.89 mg GAE/100 g db and the antioxidant activities based on DPPH and ABTS assays in the ranges 134.24–137.15 and 302.80–311.85 mg·TE/100 g db, respectively. -

Effect of Preparation Method on Chemical Property of Different Thai Rice Variety
Journal of Food and Nutrition Research, 2019, Vol. 7, No. 3, 231-236 Available online at http://pubs.sciepub.com/jfnr/7/3/8 Published by Science and Education Publishing DOI:10.12691/jfnr-7-3-8 Effect of Preparation Method on Chemical Property of Different Thai Rice Variety Cahyuning Isnaini1, Pattavara Pathomrungsiyounggul2, Nattaya Konsue1,* 1Food Science and Technology Program, School of Agro-Industry, Mae Fah Luang University, Muang, Chiang Rai 57100, Thailand 2Faculty of Agro-Industry, Chiang Mai University, Muang, Chiang Mai 50100, Thailand *Corresponding author: [email protected] Received January 15, 2019; Revised February 20, 2019; Accepted March 19, 2019 Abstract Improving benefits and reducing risk of staple food consumption are of interest among researchers nowadays. Rice is the major staple foods consumed in Asia. It has been reported that rice consumption has a positive association with the risk of chronic diseases. The effects of rice variety and preparation process on chemical characteristics of rice were investigated in the current study. Three Thai rice varieties, Khao Dok Mali 105 (KDML 105), Sao Hai (SH) and Riceberry (RB), underwent parboiling or non-parboiling as well as polishing or non- polishing prior to chemical property analysis. It was found that parboiling process possessed greater content of mineral as indicated by ash content as well as fiber and total phenolic content (TPC) and 2,2-diphenyl-1- picrylhydrazyl (DPPH) radical scavenging activity when compared to non-parboiling treatments, whereas the reduction in amylose and TAC content, GI value and starch digestibility were observed in this sample. On the other hand, polishing process led to reduction in ash, amylose, fiber, TPC and TAC content and DPPH values. -
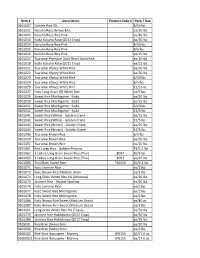
Item # Description Product Code # Pack / Size 0101007 Calrose Rice
Item # Description Product Code # Pack / Size 0101007 Calrose Rice 4% 4/10 lbs 0101011 Kokuho Rose Brown Rice ea/20 lbs 0101012 Kokuho Rose Rice Pink ea/40 lbs 0101013 Koda Kokuho Rose (2011 Crop) ea/20 lbs 0101014 Kokuho Rose Rice Pink 4/10 lbs 0101015 Kokuho Rose Rice Pink 8/5 lbs 0101016 Kokuho Rose Rice Pink ea/15 lbs 0101017 Tsurumai Premium Gold Short Grain Rice ea/20 lbs 0101018 Koda Kokuho Rose (2012 Crop) ea/15 lbs 0101021 Tsurumai Xfancy White Rice ea/50 lbs 0101022 Tsurumai Xfancy White Rice ea/20 lbs 0101023 Tsurumai Xfancy White Rice 4/10 lbs 0101024 Tsurumai Xfancy White Rice 8/5 lbs 0101025 Tsurumai Xfancy White Rice 12/16 oz 0101027 Koto Long Grain 4% White Rice ea/5 lbs 0101029 Sweet Rice Mochigome - Koda ea/50 lbs 0101030 Sweet Rice Mochigome - Koda ea/25 lbs 0101031 Sweet Rice Mochigome - Koda 6/10 lbs 0101032 Sweet Rice Mochigome - Koda 12/5 lbs 0101041 Sweet Rice (White) - Golden Crane ea/25 lbs 0101042 Sweet Rice (White) - Golden Crane 12/5 lbs 0101043 Sweet Rice (Brown) - Golden Crane ea/25 lbs 0101044 Sweet Rice (Brown) - Golden Crane 12/5 lbs 0101046 Tsurumai Brown Rice 6/5 lbs 0101050 Tsurumai Brown Rice ea/50 lbs 0101051 Tsurumai Brown Rice ea/20 lbs 0101061 Red Cargo Rice - Golden Phoenix 15/2.2 lbs 0101062 3 Ladies Long Grain Sweet Rice (Thai) 8094 10/5 lbs 0101063 3 Ladies Long Grain Sweet Rice (Thai) 8092 ea/25 lbs 0101066 Thai Black Sweet Rice TL0234 10/2.2 lbs 0101071 Koto Jasmine Rice ea/2 lbs 0101072 Koto Brown Rice Medium Grain ea/2 lbs 0101074 Long Grain White Rice 4% (Arkansas) ea/50 lbs 0101075 Jasmine -

Fumonisin Monitoring in Thai Red Cargo Rice by Reversed-Phase High-Performance Liquid Chromatography with Electrospray Ionization Ion Trap Mass Spectrometry
International Food Research Journal 19(4): 1561-1566 (2012) Journal homepage: http://www.ifrj.upm.edu.my Fumonisin monitoring in Thai red cargo rice by reversed-phase high-performance liquid chromatography with electrospray ionization ion trap mass spectrometry 1,*Tansakul, N., 2Limsuwan, S. and 1Trongvanichnam, K. 1Department of Pharmacology, Faculty of Veterinary Medicine, Kasetsart University, Chatuchak, Bangkok, 10900, Thailand 2Molecular Phytopathology and Mycotoxin Research, Department of Crop Sciences, Göttingen University, Greisebachstrasse 6, 37077, Göttingen, Germany Article history Abstract Received: 1 December 2011 The occurrence of fumonisins in red cargo rice from Thailand was studied by high-performance Received in revised form: liquid chromatography with electrospray ionization ion trap mass spectrometry (LC-ESI-MS/ 7 March 2012 MS). A quantification method for fumonisin B1 (FB1) was developed and the chromatogram Accepted:7 March 2012 of fumonisin B2 (FB2) was observed. The present method provides a sensitive detection limit at 1.0 ng g-1. The limit of quantification was 5.0 ng g-1. The recovery rate showed high yield of Keywords accuracy at 110.1±13.3, 89.3±11.1 and 91.9±4.6 % after fortification (n=5) at 50, 100 and 500 -1 Fumonisin B1, ng g , respectively. Of the fifty eight samples from the retail markets, two samples were found -1 Fumonisin B2, to be naturally contaminated with FB1 at a trace level (lower than 5.0 ng g ). None of FB2 was red cargo rice, found in any of the samples. This is the first report about the natural occurrence of FB in red mycotoxins, cargo rice from Thai market. -

Degruyter Revac Revac-2021-0137 272..292 ++
Reviews in Analytical Chemistry 2021; 40: 272–292 Review Article Vinita Ramtekey*, Susmita Cherukuri, Kaushalkumar Gunvantray Modha, Ashutosh Kumar*, Udaya Bhaskar Kethineni, Govind Pal, Arvind Nath Singh, and Sanjay Kumar Extraction, characterization, quantification, and application of volatile aromatic compounds from Asian rice cultivars https://doi.org/10.1515/revac-2021-0137 crop and deposits during seed maturation. So far, litera- received December 31, 2020; accepted May 30, 2021 ture has been focused on reporting about aromatic com- Abstract: Rice is the main staple food after wheat for pounds in rice but its extraction, characterization, and fi more than half of the world’s population in Asia. Apart quanti cation using analytical techniques are limited. from carbohydrate source, rice is gaining significant Hence, in the present review, extraction, characterization, - interest in terms of functional foods owing to the presence and application of aromatic compound have been eluci of aromatic compounds that impart health benefits by dated. These VACs can give a new way to food processing fl - lowering glycemic index and rich availability of dietary and beverage industry as bio avor and bioaroma com fibers. The demand for aromatic rice especially basmati pounds that enhance value addition of beverages, food, - rice is expanding in local and global markets as aroma is and fermented products such as gluten free rice breads. considered as the best quality and desirable trait among Furthermore, owing to their nutritional values these VACs fi consumers. There are more than 500 volatile aromatic com- can be used in bioforti cation that ultimately addresses the pounds (VACs) vouched for excellent aroma and flavor in food nutrition security. -

Potential Anti-Inflammatory and Anti-Oxidative Properties of Thai Colored-Rice Extracts
POJ 8(1):69-77 (2015) ISSN:1836-3644 Potential anti-inflammatory and anti-oxidative properties of Thai colored-rice extracts Thitinan Kitisin1, Nisakorn Saewan2, Natthanej Luplertlop3* 1Department of Anatomy, Faculty of Science, Mahidol University, Ratchathewi, Bangkok, Thailand 2School of Cosmetic Science, Mae Fah Luang University, Muang, Chiang Rai, Thailand 3Department of Microbiology and Immunology, Faculty of Tropical Medicine, Mahidol University, Ratchathewi, Bangkok, Thailand *Corresponding author: [email protected] Abstract In Thailand, there has been growing interest in the use of colored rice extracts as a new source of anti-oxidative and anti- inflammatory effects. This study investigates the effects of different colored rice extracts in terms of their biological content, anti- oxidative activity, and their ability to reduce pro-inflammatory cytokines and matrix metalloproteinase (MMP) expression. Various colored rice from different rice cultivating areas in Thailand were used to obtain ethanolic extracts. The biological compounds in colored-rice extracts were determined by Folin-Ciocalteu colorimetric and pH-differential methods. To determine the anti-oxidative properties of colored-rice extract, DPPH radical scavenging, ferrous reducing power, and lipid peroxidation assays were used. The cytotoxicity of colored rice extracts was determined by MTT assay on a human promyelocytic leukemia (HL-60) cell line in vitro. The inhibition of pro-inflammatory cytokines (IL-6, TNF-α, NF-κB) and MMP expression in LPS-induced HL-60 cells was determined by ELISA assay. Moreover, MMP activity was determined by gelatinolytic zymography. The results found that red (Mun Poo, MP) rice exhibited high anti-oxidative activity and reduced pro-inflammatory cytokines and MMP-2 expression in LPS-induced HL-60 cells. -

J I T M M 2 0
JOINT INTERNATIONAL TROPICAL MEDICINE MEETING 2018 “INNOVATION, TRANSLATION, AND IMPACT IN TROPICAL MEDICINE” 12 – 14 DECEMBER 2018 AMARI WATERGATE HOTEL, BANGKOK, THAILAND ABSTRACTS Oral Presentations J I T M M 2 0 1 8 Organizers 4 Faculty of Tropical Medicine, Mahidol University 4 SEAMEO TROPMED Network 4 TROPMED Alumni Association 4 The Parasitology and Tropical Medicine Association of Thailand Co-organizers 9 Department of Disease Control Ministry of Public Health (MOPH) 9 Mahidol - Oxford Tropical Medicine Research Unit (MORU) 2 Wednesday 12 December 2018 Opening Session 09.00-09.45 Watergate Ballroom OPENING CEREMONY BY ORGANIZERS AND CO-ORGANIZERS Report by: Prof. Srivicha Krudsood Chair, JITMM2018 Scientific Committee WELCOME ADDRESS Dr. Sombat Thanphasertsuk Senior Expert in Prevention Medicine, Department of Disease Control, Thailand Ministry of Public Health WELCOME ADDRESS Mr. David Burton Chief Operating Officer, Mahidol-Oxford Tropical Medicine Research Unit (MORU) OPENING REMARKS Assoc. Prof. Pratap Singhasivanon Chairman, JITMM2018 Organizing Committee TROPMED Alumni Award Presentation Presented by: Assoc. Prof. Supranee Changbumrung Joint International Tropical Medicine Meeting (JITMM) 2018 “INNOVATION, TRANSLATION, AND IMPACT IN TROPICAL MEDICINE” 3 AWARD RECIPIENTS: Prof. Akira Kaneko Professor of Global Health, Department of Microbiology, Tumor and Cell biology, Karolinska Institutet, Sweden Prof. Dr. Tawadchai Suppadit Vice President, Planning and Development Strategies, Walailak University, Thailand Dr. Twatchai Srestasupana Director, Maesot General Hospital, Mae Sot, Tak, Thailand Joint International Tropical Medicine Meeting (JITMM) 2018 “INNOVATION, TRANSLATION, AND IMPACT IN TROPICAL MEDICINE” 4 Wednesday 12 December 2018 09.45-10.30 Watergate Ballroom S1: The 24th Chamlong-Tranakchit Lecture Chairperson: Pratap Singhasivanon Keynote Speaker: The safe and effective radical cure of malaria Prof. -

Private Chef's Table 2019 Immerse Yourself in a Private Dining
Private Chef’s Table 2019 Immerse yourself in a private dining experience and taste the secrets of Singapore’s best home chefs Heritage Food Chef Eric Teo, 56 Celebrity Chef Eric Teo brings more than 30 years of culinary experience to the F&B industry in Singapore. The culinary line-up for Private Chef’s Table is specially curated by Celebrity Chef Eric Teo. In this event, Chef Eric Teo acts as a mentor to the home chefs, curating the menu and culinary themes: Heritage Food and Modern Takes, thus presenting dishes that stay true to their traditional roots and also dishes with modern interpretations. Date: 5 Jul 2019 | Time: 7-10pm Starter Cantonese Lobster & Avocado Soup Paired with Perrier-Jouët Grand Brut Teochew Heavenly King Braised Crab Meat Soup Paired with Perrier-Jouët Grand Brut Main Hokkien Kobe Beef, Bell Peppers Paired with Martell Cordon Bleu Golden Chef Abalone, King Oyster Mushroom & Asparagus Paired with Martell XO Hakka Grandma Fish Noodles Paired with Martell XO Dessert Hainan Slow Cooked Bird Nest Paired with Martell XO 1 Eat Moreish (Yun Hui, 33) As someone who lives for food experiences, Yun Hui enjoys sharing good food, fine-tuned with his own twists and style. You will find classic dishes, inspired by the cooking matriarch of the household - his grandmother. “Eat Moreish” features nosh that are zhng-ed up with a personal touch, fine-tuned with many trials and errors. The item on the menu Yun Hui is most proud of would be the Lor Bak Rice, with pork belly teased on low heat for maximum flavour. -
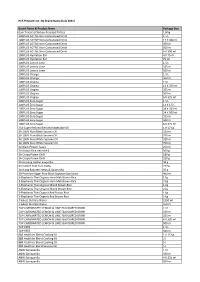
HCS Website List As of 30 June 2021 Working File.Xlsx
HCS Product List - By Brand Name (July 2021) Brand Name & Product Name Package Size (Lim Traders) Chicken Breaded Patties 1.8 kg 100PLUS ACTIVE Non-Carbonated Drink 1.5 L 100PLUS ACTIVE Non-Carbonated Drink 12 X 300 ml 100PLUS ACTIVE Non-Carbonated Drink 300 ml 100PLUS ACTIVE Non-Carbonated Drink 500 ml 100PLUS ACTIVE Non-Carbonated Drink 6 X 300 ml 100PLUS Hydration Bar 4 X 75 ml 100PLUS Hydration Bar 75 ml 100PLUS Lemon Lime 1.5 L 100PLUS Lemon Lime 325 ml 100PLUS Lemon Lime 500 ml 100PLUS Orange 1.5 L 100PLUS Orange 500 ml 100PLUS Original 1.5 L 100PLUS Original 12 X 325 ml 100PLUS Original 325 ml 100PLUS Original 500 ml 100PLUS Original 6 X 325 ml 100PLUS Zero Sugar 1.5 L 100PLUS Zero Sugar 12 X 1.5 L 100PLUS Zero Sugar 24 X 325 ml 100PLUS Zero Sugar 24 X 500 ml 100PLUS Zero Sugar 325 ml 100PLUS Zero Sugar 500 ml 100PLUS Zero Sugar 6 X 325 ml 333 Super Refined Blended Vegetable Oil 1 X 17 kg 3A 100% Pure Black Sesame Oil 320 ml 3A 100% Pure Black Sesame Oil 750 ml 3A 100% Pure White Sesame Oil 320 ml 3A 100% Pure White Sesame Oil 750 ml 3A Black Pepper Sauce 250 ml 3A Brown Rice Vermicelli 500 g 3A Crispy Prawn Chilli 180 g 3A Crispy Prawn Chilli 320 g 3A Ginseng Herbal Soup Mix 40 g 3A Instant Tom Yum Paste 227 g 3A Klang Bakuteh Herbs & Spices Mix 35 g 3A Premium Sugar Free Black Soybean Soy Sauce 400 ml 3-Elephants Thai Organic Hom Mali Brown Rice 1 kg 3-Elephants Thai Organic Hom Mali Brown Rice 2 kg 3-Elephants Thai Organic Mixed Brown Rice 1 kg 3-Elephants Thai Organic Mixed Brown Rice 2 kg 3-Elephants Thai Organic Red Brown -

2021) Journal Homepage
International Food Research Journal 28(2): 386 - 392 (April 2021) Journal homepage: http://www.ifrj.upm.edu.my Optimisation of the dielectric barrier discharge to produce Riceberry rice flour retained with high activities of bioactive compounds using plasma technology 1Settapramote, N., 2,5Laokuldilok, T., 3Boonyawan, D. and 4,5*Utama-ang, N. 1Division of Product Development Technology, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand 2Division of Marine Product Technology, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand 3Plasma and Beam Physics Research Facility, Department of Physics and Materials Science, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand 4Division of Product Development Technology, Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand 5Cluster of High Value Product from Thai Rice for Health, Chiang Mai University, Chiang Mai 50100, Thailand Article history Abstract Received: 3 December 2019 Riceberry rice is a hybrid rice that contains polyphenol compounds, anthocyanin, and high Received in revised form: antioxidants. Plasma technology has been used to improve the quality of rice and rice flour. 2 April 2020 Some conditions of the plasma process can be altered to get the combination that can achieve Accepted: 7 May 2020 maximum result. The present work aimed to identify the optimal combination of a plasma treatment condition by varying three variables: time (3 - 10 min), power (140 - 180 W), and oxygen flow rate (0.0 - 0.8 L/min) in improving the nutrient and antioxidant agent of Keywords Riceberry rice flour. The increase in time and power significantly increased the percenatge of dielectric barrier the scavenging ability of the free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH), peonidin discharge (DBD), 3-glucoside, cyanidin 3-glucoside, and total anthocyanin; while the decrease in oxygen plasma technology, significantly decreased all the parameters analysed.