Revisiting the Derivation of Heisenberg's Uncertainty Principle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture 9, P 1 Lecture 9: Introduction to QM: Review and Examples
Lecture 9, p 1 Lecture 9: Introduction to QM: Review and Examples S1 S2 Lecture 9, p 2 Photoelectric Effect Binding KE=⋅ eV = hf −Φ energy max stop Φ The work function: 3.5 Φ is the minimum energy needed to strip 3 an electron from the metal. 2.5 2 (v) Φ is defined as positive . 1.5 stop 1 Not all electrons will leave with the maximum V f0 kinetic energy (due to losses). 0.5 0 0 5 10 15 Conclusions: f (x10 14 Hz) • Light arrives in “packets” of energy (photons ). • Ephoton = hf • Increasing the intensity increases # photons, not the photon energy. Each photon ejects (at most) one electron from the metal. Recall: For EM waves, frequency and wavelength are related by f = c/ λ. Therefore: Ephoton = hc/ λ = 1240 eV-nm/ λ Lecture 9, p 3 Photoelectric Effect Example 1. When light of wavelength λ = 400 nm shines on lithium, the stopping voltage of the electrons is Vstop = 0.21 V . What is the work function of lithium? Lecture 9, p 4 Photoelectric Effect: Solution 1. When light of wavelength λ = 400 nm shines on lithium, the stopping voltage of the electrons is Vstop = 0.21 V . What is the work function of lithium? Φ = hf - eV stop Instead of hf, use hc/ λ: 1240/400 = 3.1 eV = 3.1eV - 0.21eV For Vstop = 0.21 V, eV stop = 0.21 eV = 2.89 eV Lecture 9, p 5 Act 1 3 1. If the workfunction of the material increased, (v) 2 how would the graph change? stop a. -
![Arxiv:0809.1003V5 [Hep-Ph] 5 Oct 2010 Htnadgaio Aslimits Mass Graviton and Photon I.Scr N Pcltv Htnms Limits Mass Photon Speculative and Secure III](https://docslib.b-cdn.net/cover/5435/arxiv-0809-1003v5-hep-ph-5-oct-2010-htnadgaio-aslimits-mass-graviton-and-photon-i-scr-n-pcltv-htnms-limits-mass-photon-speculative-and-secure-iii-385435.webp)
Arxiv:0809.1003V5 [Hep-Ph] 5 Oct 2010 Htnadgaio Aslimits Mass Graviton and Photon I.Scr N Pcltv Htnms Limits Mass Photon Speculative and Secure III
October 7, 2010 Photon and Graviton Mass Limits Alfred Scharff Goldhaber∗,† and Michael Martin Nieto† ∗C. N. Yang Institute for Theoretical Physics, SUNY Stony Brook, NY 11794-3840 USA and †Theoretical Division (MS B285), Los Alamos National Laboratory, Los Alamos, NM 87545 USA Efforts to place limits on deviations from canonical formulations of electromagnetism and gravity have probed length scales increasing dramatically over time. Historically, these studies have passed through three stages: (1) Testing the power in the inverse-square laws of Newton and Coulomb, (2) Seeking a nonzero value for the rest mass of photon or graviton, (3) Considering more degrees of freedom, allowing mass while preserving explicit gauge or general-coordinate invariance. Since our previous review the lower limit on the photon Compton wavelength has improved by four orders of magnitude, to about one astronomical unit, and rapid current progress in astronomy makes further advance likely. For gravity there have been vigorous debates about even the concept of graviton rest mass. Meanwhile there are striking observations of astronomical motions that do not fit Einstein gravity with visible sources. “Cold dark matter” (slow, invisible classical particles) fits well at large scales. “Modified Newtonian dynamics” provides the best phenomenology at galactic scales. Satisfying this phenomenology is a requirement if dark matter, perhaps as invisible classical fields, could be correct here too. “Dark energy” might be explained by a graviton-mass-like effect, with associated Compton wavelength comparable to the radius of the visible universe. We summarize significant mass limits in a table. Contents B. Einstein’s general theory of relativity and beyond? 20 C. -

The Uncertainty Principle (Stanford Encyclopedia of Philosophy) Page 1 of 14
The Uncertainty Principle (Stanford Encyclopedia of Philosophy) Page 1 of 14 Open access to the SEP is made possible by a world-wide funding initiative. Please Read How You Can Help Keep the Encyclopedia Free The Uncertainty Principle First published Mon Oct 8, 2001; substantive revision Mon Jul 3, 2006 Quantum mechanics is generally regarded as the physical theory that is our best candidate for a fundamental and universal description of the physical world. The conceptual framework employed by this theory differs drastically from that of classical physics. Indeed, the transition from classical to quantum physics marks a genuine revolution in our understanding of the physical world. One striking aspect of the difference between classical and quantum physics is that whereas classical mechanics presupposes that exact simultaneous values can be assigned to all physical quantities, quantum mechanics denies this possibility, the prime example being the position and momentum of a particle. According to quantum mechanics, the more precisely the position (momentum) of a particle is given, the less precisely can one say what its momentum (position) is. This is (a simplistic and preliminary formulation of) the quantum mechanical uncertainty principle for position and momentum. The uncertainty principle played an important role in many discussions on the philosophical implications of quantum mechanics, in particular in discussions on the consistency of the so-called Copenhagen interpretation, the interpretation endorsed by the founding fathers Heisenberg and Bohr. This should not suggest that the uncertainty principle is the only aspect of the conceptual difference between classical and quantum physics: the implications of quantum mechanics for notions as (non)-locality, entanglement and identity play no less havoc with classical intuitions. -

Path Integral for the Hydrogen Atom
Path Integral for the Hydrogen Atom Solutions in two and three dimensions Vägintegral för Väteatomen Lösningar i två och tre dimensioner Anders Svensson Faculty of Health, Science and Technology Physics, Bachelor Degree Project 15 ECTS Credits Supervisor: Jürgen Fuchs Examiner: Marcus Berg June 2016 Abstract The path integral formulation of quantum mechanics generalizes the action principle of classical mechanics. The Feynman path integral is, roughly speaking, a sum over all possible paths that a particle can take between fixed endpoints, where each path contributes to the sum by a phase factor involving the action for the path. The resulting sum gives the probability amplitude of propagation between the two endpoints, a quantity called the propagator. Solutions of the Feynman path integral formula exist, however, only for a small number of simple systems, and modifications need to be made when dealing with more complicated systems involving singular potentials, including the Coulomb potential. We derive a generalized path integral formula, that can be used in these cases, for a quantity called the pseudo-propagator from which we obtain the fixed-energy amplitude, related to the propagator by a Fourier transform. The new path integral formula is then successfully solved for the Hydrogen atom in two and three dimensions, and we obtain integral representations for the fixed-energy amplitude. Sammanfattning V¨agintegral-formuleringen av kvantmekanik generaliserar minsta-verkanprincipen fr˚anklassisk meka- nik. Feynmans v¨agintegral kan ses som en summa ¨over alla m¨ojligav¨agaren partikel kan ta mellan tv˚a givna ¨andpunkterA och B, d¨arvarje v¨agbidrar till summan med en fasfaktor inneh˚allandeden klas- siska verkan f¨orv¨agen.Den resulterande summan ger propagatorn, sannolikhetsamplituden att partikeln g˚arfr˚anA till B. -
Compton Effect
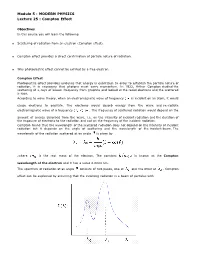
Module 5 : MODERN PHYSICS Lecture 25 : Compton Effect Objectives In this course you will learn the following Scattering of radiation from an electron (Compton effect). Compton effect provides a direct confirmation of particle nature of radiation. Why photoelectric effect cannot be exhited by a free electron. Compton Effect Photoelectric effect provides evidence that energy is quantized. In order to establish the particle nature of radiation, it is necessary that photons must carry momentum. In 1922, Arthur Compton studied the scattering of x-rays of known frequency from graphite and looked at the recoil electrons and the scattered x-rays. According to wave theory, when an electromagnetic wave of frequency is incident on an atom, it would cause electrons to oscillate. The electrons would absorb energy from the wave and re-radiate electromagnetic wave of a frequency . The frequency of scattered radiation would depend on the amount of energy absorbed from the wave, i.e. on the intensity of incident radiation and the duration of the exposure of electrons to the radiation and not on the frequency of the incident radiation. Compton found that the wavelength of the scattered radiation does not depend on the intensity of incident radiation but it depends on the angle of scattering and the wavelength of the incident beam. The wavelength of the radiation scattered at an angle is given by .where is the rest mass of the electron. The constant is known as the Compton wavelength of the electron and it has a value 0.0024 nm. The spectrum of radiation at an angle consists of two peaks, one at and the other at . -

Schrodinger's Uncertainty Principle? Lilies Can Be Painted
Rajaram Nityananda Schrodinger's Uncertainty Principle? lilies can be Painted Rajaram Nityananda The famous equation of quantum theory, ~x~px ~ h/47r = 11,/2 is of course Heisenberg'S uncertainty principlel ! But SchrO dinger's subsequent refinement, described in this article, de serves to be better known in the classroom. Rajaram Nityananda Let us recall the basic algebraic steps in the text book works at the Raman proof. We consider the wave function (which has a free Research Institute,· real parameter a) (x + iap)1jJ == x1jJ(x) + ia( -in81jJ/8x) == mainly on applications of 4>( x), The hat sign over x and p reminds us that they are optical and statistical operators. We have dropped the suffix x on the momentum physics, for eJ:ample in p but from now on, we are only looking at its x-component. astronomy. Conveying 1jJ( x) is physics to students at Even though we know nothing about except that it different levels is an allowed wave function, we can be sure that J 4>* ¢dx ~ O. another activity. He In terms of 1jJ, this reads enjoys second class rail travel, hiking, ! 1jJ*(x - iap)(x + iap)1jJdx ~ O. (1) deciphering signboards in strange scripts and Note the all important minus sign in the first bracket, potatoes in any form. coming from complex conjugation. The product of operators can be expanded and the result reads2 < x2 > + < a2p2 > +ia < (xp - px) > ~ O. (2) I I1x is the uncertainty in the x The three terms are the averages of (i) the square of component of position and I1px the uncertainty in the x the coordinate, (ii) the square of the momentum, (iii) the component of the momentum "commutator" xp - px. -

1 the Principle of Wave–Particle Duality: an Overview
3 1 The Principle of Wave–Particle Duality: An Overview 1.1 Introduction In the year 1900, physics entered a period of deep crisis as a number of peculiar phenomena, for which no classical explanation was possible, began to appear one after the other, starting with the famous problem of blackbody radiation. By 1923, when the “dust had settled,” it became apparent that these peculiarities had a common explanation. They revealed a novel fundamental principle of nature that wascompletelyatoddswiththeframeworkofclassicalphysics:thecelebrated principle of wave–particle duality, which can be phrased as follows. The principle of wave–particle duality: All physical entities have a dual character; they are waves and particles at the same time. Everything we used to regard as being exclusively a wave has, at the same time, a corpuscular character, while everything we thought of as strictly a particle behaves also as a wave. The relations between these two classically irreconcilable points of view—particle versus wave—are , h, E = hf p = (1.1) or, equivalently, E h f = ,= . (1.2) h p In expressions (1.1) we start off with what we traditionally considered to be solely a wave—an electromagnetic (EM) wave, for example—and we associate its wave characteristics f and (frequency and wavelength) with the corpuscular charac- teristics E and p (energy and momentum) of the corresponding particle. Conversely, in expressions (1.2), we begin with what we once regarded as purely a particle—say, an electron—and we associate its corpuscular characteristics E and p with the wave characteristics f and of the corresponding wave. -

Zero-Point Energy and Interstellar Travel by Josh Williams
;;;;;;;;;;;;;;;;;;;;;; itself comes from the conversion of electromagnetic Zero-Point Energy and radiation energy into electrical energy, or more speciÞcally, the conversion of an extremely high Interstellar Travel frequency bandwidth of the electromagnetic spectrum (beyond Gamma rays) now known as the zero-point by Josh Williams spectrum. ÒEre many generations pass, our machinery will be driven by power obtainable at any point in the universeÉ it is a mere question of time when men will succeed in attaching their machinery to the very wheel work of nature.Ó ÐNikola Tesla, 1892 Some call it the ultimate free lunch. Others call it absolutely useless. But as our world civilization is quickly coming upon a terrifying energy crisis with As you can see, the wavelengths from this part of little hope at the moment, radical new ideas of usable the spectrum are incredibly small, literally atomic and energy will be coming to the forefront and zero-point sub-atomic in size. And since the wavelength is so energy is one that is currently on its way. small, it follows that the frequency is quite high. The importance of this is that the intensity of the energy So, what the hell is it? derived for zero-point energy has been reported to be Zero-point energy is a type of energy that equal to the cube (the third power) of the frequency. exists in molecules and atoms even at near absolute So obviously, since weÕre already talking about zero temperatures (-273¡C or 0 K) in a vacuum. some pretty high frequencies with this portion of At even fractions of a Kelvin above absolute zero, the electromagnetic spectrum, weÕre talking about helium still remains a liquid, and this is said to be some really high energy intensities. -

The Graviton Compton Mass As Dark Energy
Gravitation, Mathematical Physics and Field Theory Revista Mexicana de F´ısica 67 040703 1–5 JULY-AUGUST 2021 The graviton Compton mass as dark energy T. Matosa and L. L.-Parrillab aDepartamento de F´ısica, Centro de Investigacion´ y de Estudios Avanzados del IPN, Apartado Postal 14-740, 07000, CDMX, Mexico.´ bInstituto de Ciencias Nucleares, Universidad Nacional Autonoma´ de Mexico,´ Circuito Exterior C.U., Apartado Postal 70-543, 04510, CDMX, Mexico.´ Received 3 January 2021; accepted 18 February 2021 One of the greatest challenges of science is to understand the current accelerated expansion of the Universe. In this work, we show that by considering the quantum nature of the gravitational field, its wavelength can be associated with an effective Compton mass. We propose that this mass can be interpreted as dark energy, with a Compton wavelength given by the size of the observable Universe, implying that the dark energy varies depending on this size. If we do so, we find that: 1.- Even without any free constant for dark energy, the evolution of the Hubble parameter is exactly the same as for the LCDM model, so this model has the same predictions as LCDM. 2.- The density rate of the dark energy is ¤ = 0:69 which is a very similar value as the one found by the Planck satellite ¤ = 0:684. 3.- The dark energy has this value because it corresponds to the actual size of the radius of the Universe, thus the coincidence problem has a very natural explanation. 4.- It, is possible to find also a natural explanation to why observations inferred from the local distance ladder find the value H0 = 73 km/s/Mpc for the Hubble constant. -

The Paradoxes of the Interaction-Free Measurements
The Paradoxes of the Interaction-free Measurements L. Vaidman Centre for Quantum Computation, Department of Physics, University of Oxford, Clarendon Laboratory, Parks Road, Oxford 0X1 3PU, England; School of Physics and Astronomy, Raymond and Beverly Sackler Faculty of Exact Sciences, Tel-Aviv University, Tel-Aviv 69978, Israel Reprint requests to Prof. L. V.; E-mail: [email protected] Z. Naturforsch. 56 a, 100-107 (2001); received January 12, 2001 Presented at the 3rd Workshop on Mysteries, Puzzles and Paradoxes in Quantum Mechanics, Gargnano, Italy, September 17-23, 2000. Interaction-free measurements introduced by Elitzur and Vaidman [Found. Phys. 23,987 (1993)] allow finding infinitely fragile objects without destroying them. Paradoxical features of these and related measurements are discussed. The resolution of the paradoxes in the framework of the Many-Worlds Interpretation is proposed. Key words: Interaction-free Measurements; Quantum Paradoxes. I. Introduction experiment” proposed by Wheeler [22] which helps to define the context in which the above claims, that The interaction-free measurements proposed by the measurements are interaction-free, are legitimate. Elitzur and Vaidman [1,2] (EVIFM) led to numerousSection V is devoted to the variation of the EV IFM investigations and several experiments have been per proposed by Penrose [23] which, instead of testing for formed [3- 17]. Interaction-free measurements are the presence of an object in a particular place, tests very paradoxical. Usually it is claimed that quantum a certain property of the object in an interaction-free measurements, in contrast to classical measurements, way. Section VI introduces the EV IFM procedure for invariably cause a disturbance of the system. -

Quantum Certainty Mechanics
Quantum Certainty Mechanics Muhammad Yasin Savar, Dhaka, Bangladesh E-mail:[email protected] 1 Abstract: Quantum certainty mechanics is a theory for measuring the position and momentum of a particle. Mathematically proven certainty principle from uncertainty principle, which is basically one of the most important formulas of quantum certainty mechanics theory. The principle of uncertainty can be proved by the principle of certainty and why uncertainty comes can also be proved. The principle of certainty can be proved from the theory of relativity and in the uncertainty principle equation, the principle of certainty can be proved by fulfilling the conditions of the principle of uncertainty by multiplying the uncertain constant with the certain values of momentum-position and energy-time. The principle of certainty proves that the calculation of θ ≥π/2 between the particle and the wave involved in the particle leads to uncertainty. But calculating with θ=0 does not bring uncertainty. Again, if the total energy E of the particle is measured accurately in the laboratory, the momentum and position can be measured with certainty. Quantum certainty mechanics has been established by combining Newtonian mechanics, relativity theory and quantum mechanics. Quantum entanglement can be explained by protecting the conservation law of energy. 2 Keywords: quantum mechanics; uncertainty principle; quantum entanglement; Planck’s radiation law ; bohr's atomic model; photoelectric effect; certainty mechanics ; quantum measurement. 3 Introduction: In 1927 Heisenberg has invented the uncertainty of principle. The principle of uncertainty is, "It is impossible to determine the position and momentum of a particle at the same time."The more accurately the momentum is measured, the more uncertain the position will be. -

Photon Wave Mechanics: a De Broglie - Bohm Approach
PHOTON WAVE MECHANICS: A DE BROGLIE - BOHM APPROACH S. ESPOSITO Dipartimento di Scienze Fisiche, Universit`adi Napoli “Federico II” and Istituto Nazionale di Fisica Nucleare, Sezione di Napoli Mostra d’Oltremare Pad. 20, I-80125 Napoli Italy e-mail: [email protected] Abstract. We compare the de Broglie - Bohm theory for non-relativistic, scalar matter particles with the Majorana-R¨omer theory of electrodynamics, pointing out the impressive common pecu- liarities and the role of the spin in both theories. A novel insight into photon wave mechanics is envisaged. 1. Introduction Modern Quantum Mechanics was born with the observation of Heisenberg [1] that in atomic (and subatomic) systems there are directly observable quantities, such as emission frequencies, intensities and so on, as well as non directly observable quantities such as, for example, the position coordinates of an electron in an atom at a given time instant. The later fruitful developments of the quantum formalism was then devoted to connect observable quantities between them without the use of a model, differently to what happened in the framework of old quantum mechanics where specific geometrical and mechanical models were investigated to deduce the values of the observable quantities from a substantially non observable underlying structure. We now know that quantum phenomena are completely described by a complex- valued state function ψ satisfying the Schr¨odinger equation. The probabilistic in- terpretation of it was first suggested by Born [2] and, in the light of Heisenberg uncertainty principle, is a pillar of quantum mechanics itself. All the known experiments show that the probabilistic interpretation of the wave function is indeed the correct one (see any textbook on quantum mechanics, for 2 S.