Lecture 9, P 1 Lecture 9: Introduction to QM: Review and Examples
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

31 Diffraction and Interference.Pdf
Diffraction and Interference Figu These expar new .5 wave oday Isaac Newton is most famous for his new v accomplishments in mechanics—his laws of Tmotion and universal gravitation. Early in his career, however, he was most famous for his work on light. Newton pictured light as a beam of ultra-tiny material particles. With this model he could explain reflection as a bouncing of the parti- cles from a surface, and he could explain refraction as the result of deflecting forces from the surface Interference colors. acting on the light particles. In the eighteenth and nineteenth cen- turies, this particle model gave way to a wave model of light because waves could explain not only reflection and refraction, but everything else that was known about light at that time. In this chapter we will investigate the wave aspects of light, which are needed to explain two important phenomena—diffraction and interference. As Huygens' Principle origin; 31.1 pie is I constr In the late 1600s a Dutch mathematician-scientist, Christian Huygens, dimen proposed a very interesting idea about waves. Huygens stated that light waves spreading out from a point source may be regarded as die overlapping of tiny secondary wavelets, and that every point on ' any wave front may be regarded as a new point source of secondary waves. We see this in Figure 31.1. In other words, wave fronts are A made up of tinier wave fronts. This idea is called Huygens' principle. Look at the spherical wave front in Figure 31.2. Each point along the wave front AN is the source of a new wavelet that spreads out in a sphere from that point. -
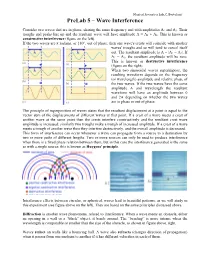
Prelab 5 – Wave Interference
Musical Acoustics Lab, C. Bertulani PreLab 5 – Wave Interference Consider two waves that are in phase, sharing the same frequency and with amplitudes A1 and A2. Their troughs and peaks line up and the resultant wave will have amplitude A = A1 + A2. This is known as constructive interference (figure on the left). If the two waves are π radians, or 180°, out of phase, then one wave's crests will coincide with another waves' troughs and so will tend to cancel itself out. The resultant amplitude is A = |A1 − A2|. If A1 = A2, the resultant amplitude will be zero. This is known as destructive interference (figure on the right). When two sinusoidal waves superimpose, the resulting waveform depends on the frequency (or wavelength) amplitude and relative phase of the two waves. If the two waves have the same amplitude A and wavelength the resultant waveform will have an amplitude between 0 and 2A depending on whether the two waves are in phase or out of phase. The principle of superposition of waves states that the resultant displacement at a point is equal to the vector sum of the displacements of different waves at that point. If a crest of a wave meets a crest of another wave at the same point then the crests interfere constructively and the resultant crest wave amplitude is increased; similarly two troughs make a trough of increased amplitude. If a crest of a wave meets a trough of another wave then they interfere destructively, and the overall amplitude is decreased. This form of interference can occur whenever a wave can propagate from a source to a destination by two or more paths of different lengths. -

Waves: Interference Instructions: Read Through the Information Below
Pre-AP Physics Week 2: Day 1 Notes Waves: Interference Instructions: Read through the information below. Then answer the questions below. When two or more waves meet, they interact with each other. The interaction of waves with other waves is called wave interference. Wave interference may occur when two waves that are traveling in opposite directions meet. The two waves pass through each other, and this affects their amplitude. Amplitude is the maximum distance the particles of the medium move from their resting positions when a wave passes through. How amplitude is affected by wave interference depends on the type of interference. Interference can be constructive or destructive. Constructive Interference Destructive Interference Constructive interference occurs when crests of one Destructive interference occurs when the crests of one wave overlap the crests of the other wave. The figure wave overlap the troughs, or lowest points, of another above shows what happens. As the waves pass through wave. The figure above shows what happens. As the each other, the crests combine to produce a wave with waves pass through each other, the crests and troughs greater amplitude. The same can happen with two cancel each other out to produce a wave with zero troughs interacting. amplitude. (NOTE: They don’t always have to fully cancel out. Destructive interference can be partial cancellation.) TRUE or FALSE: Identify the following statements as being either true (T) or false (F). 1. When two pulses meet up with each other while moving through the same medium, they tend to bounce off each other and return back to their origin. -

Time-Domain Grating with a Periodically Driven Qutrit
PHYSICAL REVIEW APPLIED 11, 014053 (2019) Time-Domain Grating with a Periodically Driven Qutrit Yingying Han,1,2,3,§ Xiao-Qing Luo,2,3,§ Tie-Fu Li,4,2,* Wenxian Zhang,1,† Shuai-Peng Wang,2 J.S. Tsai,5,6 Franco Nori,5,7 and J.Q. You3,2,‡ 1 School of Physics and Technology, Wuhan University, Wuhan, Hubei 430072, China 2 Quantum Physics and Quantum Information Division, Beijing Computational Science Research Center, Beijing 100193, China 3 Interdisciplinary Center of Quantum Information and Zhejiang Province Key Laboratory of Quantum Technology and Device, Department of Physics and State Key Laboratory of Modern Optical Instrumentation, Zhejiang University, Hangzhou 310027, China 4 Institute of Microelectronics, Department of Microelectronics and Nanoelectronics, and Tsinghua National Laboratory of Information Science and Technology, Tsinghua University, Beijing 100084, China 5 Theoretical Quantum Physics Laboratory, RIKEN Cluster for Pioneering Research, Wako-shi, Saitama 351-0198, Japan 6 Department of Physic, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan 7 Department of Physics, The University of Michigan, Ann Arbor, Michigan 48109-1040, USA (Received 23 October 2018; revised manuscript received 3 December 2018; published 28 January 2019) Physical systems in the time domain may exhibit analogous phenomena in real space, such as time crystals, time-domain Fresnel lenses, and modulational interference in a qubit. Here, we report the experi- mental realization of time-domain grating using a superconducting qutrit in periodically modulated probe and control fields via two schemes: simultaneous modulation and complementary modulation. Both exper- imental and numerical results exhibit modulated Autler-Townes (AT) and modulation-induced diffraction (MID) effects. -

The Uncertainty Principle (Stanford Encyclopedia of Philosophy) Page 1 of 14
The Uncertainty Principle (Stanford Encyclopedia of Philosophy) Page 1 of 14 Open access to the SEP is made possible by a world-wide funding initiative. Please Read How You Can Help Keep the Encyclopedia Free The Uncertainty Principle First published Mon Oct 8, 2001; substantive revision Mon Jul 3, 2006 Quantum mechanics is generally regarded as the physical theory that is our best candidate for a fundamental and universal description of the physical world. The conceptual framework employed by this theory differs drastically from that of classical physics. Indeed, the transition from classical to quantum physics marks a genuine revolution in our understanding of the physical world. One striking aspect of the difference between classical and quantum physics is that whereas classical mechanics presupposes that exact simultaneous values can be assigned to all physical quantities, quantum mechanics denies this possibility, the prime example being the position and momentum of a particle. According to quantum mechanics, the more precisely the position (momentum) of a particle is given, the less precisely can one say what its momentum (position) is. This is (a simplistic and preliminary formulation of) the quantum mechanical uncertainty principle for position and momentum. The uncertainty principle played an important role in many discussions on the philosophical implications of quantum mechanics, in particular in discussions on the consistency of the so-called Copenhagen interpretation, the interpretation endorsed by the founding fathers Heisenberg and Bohr. This should not suggest that the uncertainty principle is the only aspect of the conceptual difference between classical and quantum physics: the implications of quantum mechanics for notions as (non)-locality, entanglement and identity play no less havoc with classical intuitions. -

Path Integral for the Hydrogen Atom
Path Integral for the Hydrogen Atom Solutions in two and three dimensions Vägintegral för Väteatomen Lösningar i två och tre dimensioner Anders Svensson Faculty of Health, Science and Technology Physics, Bachelor Degree Project 15 ECTS Credits Supervisor: Jürgen Fuchs Examiner: Marcus Berg June 2016 Abstract The path integral formulation of quantum mechanics generalizes the action principle of classical mechanics. The Feynman path integral is, roughly speaking, a sum over all possible paths that a particle can take between fixed endpoints, where each path contributes to the sum by a phase factor involving the action for the path. The resulting sum gives the probability amplitude of propagation between the two endpoints, a quantity called the propagator. Solutions of the Feynman path integral formula exist, however, only for a small number of simple systems, and modifications need to be made when dealing with more complicated systems involving singular potentials, including the Coulomb potential. We derive a generalized path integral formula, that can be used in these cases, for a quantity called the pseudo-propagator from which we obtain the fixed-energy amplitude, related to the propagator by a Fourier transform. The new path integral formula is then successfully solved for the Hydrogen atom in two and three dimensions, and we obtain integral representations for the fixed-energy amplitude. Sammanfattning V¨agintegral-formuleringen av kvantmekanik generaliserar minsta-verkanprincipen fr˚anklassisk meka- nik. Feynmans v¨agintegral kan ses som en summa ¨over alla m¨ojligav¨agaren partikel kan ta mellan tv˚a givna ¨andpunkterA och B, d¨arvarje v¨agbidrar till summan med en fasfaktor inneh˚allandeden klas- siska verkan f¨orv¨agen.Den resulterande summan ger propagatorn, sannolikhetsamplituden att partikeln g˚arfr˚anA till B. -

Physical Quantum States and the Meaning of Probability Michel Paty
Physical quantum states and the meaning of probability Michel Paty To cite this version: Michel Paty. Physical quantum states and the meaning of probability. Galavotti, Maria Carla, Suppes, Patrick and Costantini, Domenico. Stochastic Causality, CSLI Publications (Center for Studies on Language and Information), Stanford (Ca, USA), p. 235-255, 2001. halshs-00187887 HAL Id: halshs-00187887 https://halshs.archives-ouvertes.fr/halshs-00187887 Submitted on 15 Nov 2007 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. as Chapter 14, in Galavotti, Maria Carla, Suppes, Patrick and Costantini, Domenico, (eds.), Stochastic Causality, CSLI Publications (Center for Studies on Language and Information), Stanford (Ca, USA), 2001, p. 235-255. Physical quantum states and the meaning of probability* Michel Paty Ëquipe REHSEIS (UMR 7596), CNRS & Université Paris 7-Denis Diderot, 37 rue Jacob, F-75006 Paris, France. E-mail : [email protected] Abstract. We investigate epistemologically the meaning of probability as implied in quantum physics in connection with a proposed direct interpretation of the state function and of the related quantum theoretical quantities in terms of physical systems having physical properties, through an extension of meaning of the notion of physical quantity to complex mathematical expressions not reductible to simple numerical values. -

Schrodinger's Uncertainty Principle? Lilies Can Be Painted
Rajaram Nityananda Schrodinger's Uncertainty Principle? lilies can be Painted Rajaram Nityananda The famous equation of quantum theory, ~x~px ~ h/47r = 11,/2 is of course Heisenberg'S uncertainty principlel ! But SchrO dinger's subsequent refinement, described in this article, de serves to be better known in the classroom. Rajaram Nityananda Let us recall the basic algebraic steps in the text book works at the Raman proof. We consider the wave function (which has a free Research Institute,· real parameter a) (x + iap)1jJ == x1jJ(x) + ia( -in81jJ/8x) == mainly on applications of 4>( x), The hat sign over x and p reminds us that they are optical and statistical operators. We have dropped the suffix x on the momentum physics, for eJ:ample in p but from now on, we are only looking at its x-component. astronomy. Conveying 1jJ( x) is physics to students at Even though we know nothing about except that it different levels is an allowed wave function, we can be sure that J 4>* ¢dx ~ O. another activity. He In terms of 1jJ, this reads enjoys second class rail travel, hiking, ! 1jJ*(x - iap)(x + iap)1jJdx ~ O. (1) deciphering signboards in strange scripts and Note the all important minus sign in the first bracket, potatoes in any form. coming from complex conjugation. The product of operators can be expanded and the result reads2 < x2 > + < a2p2 > +ia < (xp - px) > ~ O. (2) I I1x is the uncertainty in the x The three terms are the averages of (i) the square of component of position and I1px the uncertainty in the x the coordinate, (ii) the square of the momentum, (iii) the component of the momentum "commutator" xp - px. -

Zero-Point Energy and Interstellar Travel by Josh Williams
;;;;;;;;;;;;;;;;;;;;;; itself comes from the conversion of electromagnetic Zero-Point Energy and radiation energy into electrical energy, or more speciÞcally, the conversion of an extremely high Interstellar Travel frequency bandwidth of the electromagnetic spectrum (beyond Gamma rays) now known as the zero-point by Josh Williams spectrum. ÒEre many generations pass, our machinery will be driven by power obtainable at any point in the universeÉ it is a mere question of time when men will succeed in attaching their machinery to the very wheel work of nature.Ó ÐNikola Tesla, 1892 Some call it the ultimate free lunch. Others call it absolutely useless. But as our world civilization is quickly coming upon a terrifying energy crisis with As you can see, the wavelengths from this part of little hope at the moment, radical new ideas of usable the spectrum are incredibly small, literally atomic and energy will be coming to the forefront and zero-point sub-atomic in size. And since the wavelength is so energy is one that is currently on its way. small, it follows that the frequency is quite high. The importance of this is that the intensity of the energy So, what the hell is it? derived for zero-point energy has been reported to be Zero-point energy is a type of energy that equal to the cube (the third power) of the frequency. exists in molecules and atoms even at near absolute So obviously, since weÕre already talking about zero temperatures (-273¡C or 0 K) in a vacuum. some pretty high frequencies with this portion of At even fractions of a Kelvin above absolute zero, the electromagnetic spectrum, weÕre talking about helium still remains a liquid, and this is said to be some really high energy intensities. -

When the Uncertainty Principle Goes to 11... Or How to Explain Quantum Physics with Heavy Metal
Ronald E. Hatcher Science on Saturday Lecture Series 12 January 2019 When The Uncertainty Principle goes to 11... or How to Explain Quantum Physics with Heavy Metal Philip Moriarty Professor, University of Nottingham, United Kingdom ABSTRACT: There are deep and fundamental linKs between quantum physics and heavy metal. No, really. There are. Far from being music for Neanderthals, as it’s too often construed, metal can be harmonically and rhythmically complex. That complexity is the source of many connections to the quantum world. You’ll discover how the Heisenberg uncertainty principle comes into play with every chugging guitar riff, what wave interference has to do with Iron Maiden, and why metalheads in mosh pits behave just liKe molecules in a gas. BIOGRAPHY: Philip Moriarty is a professor of physics, a heavy metal fan, a Keen air-drummer, and author of “When The Uncertainty Principle Goes To 11 (or How To Explain Quantum Physics With Heavy Metal” (Ben Bella 2018). His research at the University of Nottingham focuses on prodding, pushing, and poKing single atoms and molecules; in this nanoscopic world, quantum physics is all. Moriarty has taught physics for twenty years and has always been strucK by the number of students in his classes who profess a love of metal music, and by the deep connections between heavy metal and quantum mechanics. A frequent contributor to the Sixty Symbols YouTube channel, which won the Institute of Physics’ Kelvin Award in 2016 for “for innovative and effective promotion of the public understanding of physics”, he has a Keen interest in public engagement, outreach, and bridging the arts-science divide. -

The Paradoxes of the Interaction-Free Measurements
The Paradoxes of the Interaction-free Measurements L. Vaidman Centre for Quantum Computation, Department of Physics, University of Oxford, Clarendon Laboratory, Parks Road, Oxford 0X1 3PU, England; School of Physics and Astronomy, Raymond and Beverly Sackler Faculty of Exact Sciences, Tel-Aviv University, Tel-Aviv 69978, Israel Reprint requests to Prof. L. V.; E-mail: [email protected] Z. Naturforsch. 56 a, 100-107 (2001); received January 12, 2001 Presented at the 3rd Workshop on Mysteries, Puzzles and Paradoxes in Quantum Mechanics, Gargnano, Italy, September 17-23, 2000. Interaction-free measurements introduced by Elitzur and Vaidman [Found. Phys. 23,987 (1993)] allow finding infinitely fragile objects without destroying them. Paradoxical features of these and related measurements are discussed. The resolution of the paradoxes in the framework of the Many-Worlds Interpretation is proposed. Key words: Interaction-free Measurements; Quantum Paradoxes. I. Introduction experiment” proposed by Wheeler [22] which helps to define the context in which the above claims, that The interaction-free measurements proposed by the measurements are interaction-free, are legitimate. Elitzur and Vaidman [1,2] (EVIFM) led to numerousSection V is devoted to the variation of the EV IFM investigations and several experiments have been per proposed by Penrose [23] which, instead of testing for formed [3- 17]. Interaction-free measurements are the presence of an object in a particular place, tests very paradoxical. Usually it is claimed that quantum a certain property of the object in an interaction-free measurements, in contrast to classical measurements, way. Section VI introduces the EV IFM procedure for invariably cause a disturbance of the system. -

Rethinking a Delayed Choice Quantum Eraser Experiment: a Simple Baseball Model Jeffrey H
PHYSICS ESSAYS 26, 1 (2013) Rethinking a delayed choice quantum eraser experiment: A simple baseball model Jeffrey H. Boyda) 57 Woods Road, Bethany, Connecticut 06524, USA (Received 10 June 2012; accepted 26 December 2012; published online 26 February 2013) Abstract: In a double slit experiment, you can see an interference fringe pattern or you can know which slit the photon came through, but not both. There are two diametrically opposite theories about why this happens. One is complementarity. The other is the proposal made in this article. An experiment, published in 2000 [Y.-H. Kim et al., Phys. Rev. Lett. 8, 1 (2000)] allegedly supports the idea of complementarity. With different starting assumptions, we arrive at opposite conclusions: complementarity is irrelevant to this experiment. Our assumptions come from the Theory of Elementary Waves. Waves are assumed to travel in the opposite direction: from the detector to the laser. All wave interference is located at the laser. All decisions of importance are located at the laser, not at the detectors. The “decisions” concern which of several elementary rays a photon chooses to follow (this is a probabilistic decision). If a photon is emitted in response to one such impinging ray, all wave interference is then complete. The photon will follow backwards that ray alone, with a probability of one, back to the pair of detectors from which that ray originated. Based on these assumptions, the experiment has nothing to do with complementarity. There is also no delayed choice and no quantum eraser. Conclusion: complementarity is not the only way to understand this experiment.