Dynamics, Structure, and Function Are Coupled in the Mitochondrial Matrix
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mt-Atp8 Gene in the Conplastic Mouse Strain C57BL/6J-Mtfvb/NJ on the Mitochondrial Function and Consequent Alterations to Metabolic and Immunological Phenotypes
From the Lübeck Institute of Experimental Dermatology of the University of Lübeck Director: Prof. Dr. Saleh M. Ibrahim Interplay of mtDNA, metabolism and microbiota in the pathogenesis of AIBD Dissertation for Fulfillment of Requirements for the Doctoral Degree of the University of Lübeck from the Department of Natural Sciences Submitted by Paul Schilf from Rostock Lübeck, 2016 First referee: Prof. Dr. Saleh M. Ibrahim Second referee: Prof. Dr. Stephan Anemüller Chairman: Prof. Dr. Rainer Duden Date of oral examination: 30.03.2017 Approved for printing: Lübeck, 06.04.2017 Ich versichere, dass ich die Dissertation ohne fremde Hilfe angefertigt und keine anderen als die angegebenen Hilfsmittel verwendet habe. Weder vorher noch gleichzeitig habe ich andernorts einen Zulassungsantrag gestellt oder diese Dissertation vorgelegt. ABSTRACT Mitochondria are critical in the regulation of cellular metabolism and influence signaling processes and inflammatory responses. Mitochondrial DNA mutations and mitochondrial dysfunction are known to cause a wide range of pathological conditions and are associated with various immune diseases. The findings in this work describe the effect of a mutation in the mitochondrially encoded mt-Atp8 gene in the conplastic mouse strain C57BL/6J-mtFVB/NJ on the mitochondrial function and consequent alterations to metabolic and immunological phenotypes. This work provides insights into the mutation-induced cellular adaptations that influence the inflammatory milieu and shape pathological processes, in particular focusing on autoimmune bullous diseases, which have recently been reported to be associated with mtDNA polymorphisms in the human MT-ATP8 gene. The mt-Atp8 mutation diminishes the assembly of the ATP synthase complex into multimers and decreases mitochondrial respiration, affects generation of reactive oxygen species thus leading to a shift in the metabolic balance and reduction in the energy state of the cell as indicated by the ratio ATP to ADP. -

Mutation of the Fumarase Gene in Two Siblings with Progressive Encephalopathy and Fumarase Deficiency T
Mutation of the Fumarase Gene in Two Siblings with Progressive Encephalopathy and Fumarase Deficiency T. Bourgeron,* D. Chretien,* J. Poggi-Bach, S. Doonan,' D. Rabier,* P. Letouze,I A. Munnich,* A. R6tig,* P. Landneu,* and P. Rustin* *Unite de Recherches sur les Handicaps Genetiques de l'Enfant, INSERM U393, Departement de Pediatrie et Departement de Biochimie, H6pital des Enfants-Malades, 149, rue de Sevres, 75743 Paris Cedex 15, France; tDepartement de Pediatrie, Service de Neurologie et Laboratoire de Biochimie, Hopital du Kremlin-Bicetre, France; IFaculty ofScience, University ofEast-London, UK; and IService de Pediatrie, Hopital de Dreux, France Abstract chondrial enzyme (7). Human tissue fumarase is almost We report an inborn error of the tricarboxylic acid cycle, fu- equally distributed between the mitochondria, where the en- marase deficiency, in two siblings born to first cousin parents. zyme catalyzes the reversible hydration of fumarate to malate They presented with progressive encephalopathy, dystonia, as a part ofthe tricarboxylic acid cycle, and the cytosol, where it leucopenia, and neutropenia. Elevation oflactate in the cerebro- is involved in the metabolism of the fumarate released by the spinal fluid and high fumarate excretion in the urine led us to urea cycle. The two isoenzymes have quite homologous struc- investigate the activities of the respiratory chain and of the tures. In rat liver, they differ only by the acetylation of the Krebs cycle, and to finally identify fumarase deficiency in these NH2-terminal amino acid of the cytosolic form (8). In all spe- two children. The deficiency was profound and present in all cies investigated so far, the two isoenzymes have been found to tissues investigated, affecting the cytosolic and the mitochon- be encoded by a single gene (9,10). -

Characterization of a Full-Length Infectious Clone of Bovine Foamy Virus 3026
VIROLOGICA SINICA 2014, 29 (2): 94-102 DOI 10.1007/s12250-014-3382-5 RESEARCH ARTICLE Characterization of a full-length infectious clone of bovine foamy virus 3026 * Tiejun Bing1, Hong Yu1, 2, Yue Li1, Lei Sun3, Juan Tan1, Yunqi Geng1, Wentao Qiao1 1. Key Laboratory of Molecular Microbiology and Biotechnology (Ministry of Education) and Key Laboratory of Microbial Functional Genomics (Tianjin), College of Life Sciences, Nankai University, Tianjin 300071, China; 2. Department of Molecular and Cellular Pharmacology, the Vascular Biology Institute, University of Miami School of Medicine, Miami, FL 33136, USA; 3. National Laboratory of Biomacromolecules, Center for Biological Imaging, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China The biological features of most foamy viruses (FVs) are poorly understood, including bovine foamy virus (BFV). BFV strain 3026 (BFV3026) was isolated from the peripheral blood mononuclear cells of an infected cow in Zhangjiakou, China. A full-length genomic clone of BFV3026 was obtained from BFV3026-infected cells, and it exhibited more than 99% amino acid (AA) homology to another BFV strain isolated in the USA. Upon transfection into fetal canine thymus cells, the full-length BFV3026 clone produced viral structural and auxiliary proteins, typical cytopathic effects, and virus particles. These results demonstrate that the full-length BFV3026 clone is fully infectious and can be used in further BFV3026 research. KEYWORDS bovine foamy virus; infectious clone; syncytium; electron microscopy pol, and env structural genes and additional open read- INTRODUCTION ing frames (ORFs) that are under the control of the 5′- long terminal repeat (LTR) and an internal promoter (IP) Foamy viruses (FVs) are members of Retroviridae. -

Eukaryote Cell Biology - Michelle Gehringer
FUNDAMENTALS OF BIOCHEMISTRY, CELL BIOLOGY AND BIOPHYSICS – Vol. II - Eukaryote Cell Biology - Michelle Gehringer EUKARYOTE CELL BIOLOGY Michelle Gehringer Department of Biochemistry and Microbiology, University of Port Elizabeth, South Africa Keywords: cell theory, cell diversity, eukaryote cell structure, nucleus, chromatin, DNA, organelles, mitochondria, chloroplasts, transcription, RNA, translation, ribosomes, cell cycle, interphase, mitosis, meiosis, signal transduction, growth regulation, cancer, oncogenesis. Contents 1. Introduction 1.1. The first cell 2. Origin of Eukaryotes 3. Cellular differentiation in multicellular organisms 3.1. Plants 3.2. Animals 4. Eukaryotic cell structure 5. Organization of eukaryotic cells 5.1. Plasma membrane 5.2. Extracellular matrices 5.3. Protein synthesis and transport 5.4. Cytoskeleton and movement 5.5. Nucleus 5.5.1 Genomes 5.5.2 Gene expression 5.5.3 Maintaining the genome 5.6. Organelles 6. The cell cycle 6.1. Mitosis 6.2. Meiosis 7. Regulation of cell growth 7.1. Signal transduction 7.2. Programmed cell death 7.3. CancerUNESCO – EOLSS 8. Experimental Models 8.1. Yeast SAMPLE CHAPTERS 8.2. Arabidopsis 8.3. Drosophila 8.4. The mouse 8.5. Cell culture 8.6. Separation of cellular contents 8.7. Tracing biochemical pathways 9. Future Investigations Glossary Bibliography ©Encyclopedia of Life Support Systems (EOLSS) FUNDAMENTALS OF BIOCHEMISTRY, CELL BIOLOGY AND BIOPHYSICS – Vol. II - Eukaryote Cell Biology - Michelle Gehringer Biographical Sketch Summary Cells form the basic unit of life on our planet. They are well organized systems which perform all the essential tasks of eating, respiring, replicating and excreting waste products. The first cells, which are thought to have evolved about 3.8 billion years ago, much resembled present day prokaryotes. -

Mitochondrial Protein Quality Control Mechanisms
G C A T T A C G G C A T genes Review Mitochondrial Protein Quality Control Mechanisms Pooja Jadiya * and Dhanendra Tomar * Center for Translational Medicine, Lewis Katz School of Medicine, Temple University, Philadelphia, PA 19140, USA * Correspondence: [email protected] (P.J.); [email protected] (D.T.); Tel.: +1-215-707-9144 (D.T.) Received: 29 April 2020; Accepted: 15 May 2020; Published: 18 May 2020 Abstract: Mitochondria serve as a hub for many cellular processes, including bioenergetics, metabolism, cellular signaling, redox balance, calcium homeostasis, and cell death. The mitochondrial proteome includes over a thousand proteins, encoded by both the mitochondrial and nuclear genomes. The majority (~99%) of proteins are nuclear encoded that are synthesized in the cytosol and subsequently imported into the mitochondria. Within the mitochondria, polypeptides fold and assemble into their native functional form. Mitochondria health and integrity depend on correct protein import, folding, and regulated turnover termed as mitochondrial protein quality control (MPQC). Failure to maintain these processes can cause mitochondrial dysfunction that leads to various pathophysiological outcomes and the commencement of diseases. Here, we summarize the current knowledge about the role of different MPQC regulatory systems such as mitochondrial chaperones, proteases, the ubiquitin-proteasome system, mitochondrial unfolded protein response, mitophagy, and mitochondria-derived vesicles in the maintenance of mitochondrial proteome and health. The proper understanding of mitochondrial protein quality control mechanisms will provide relevant insights to treat multiple human diseases. Keywords: mitochondria; proteome; ubiquitin; proteasome; chaperones; protease; mitophagy; mitochondrial protein quality control; mitochondria-associated degradation; mitochondrial unfolded protein response 1. Introduction Mitochondria are double membrane, dynamic, and semiautonomous organelles which have several critical cellular functions. -

Characterization of Biofilm Extracts from Two Marine Bacteria
applied sciences Article Characterization of Biofilm Extracts from Two Marine Bacteria Delphine Passerini 1, Florian Fécamp 1, Laetitia Marchand 1, Laetitia Kolypczuk 1 , Sandrine Bonnetot 1, Corinne Sinquin 1 ,Véronique Verrez-Bagnis 1 , Dominique Hervio-Heath 2 , Sylvia Colliec-Jouault 1 and Christine Delbarre-Ladrat 1,* 1 Ifremer, Atlantique Center, Microbial Ecosystems and Marine Molecules for the Biotechnologies, 44311 Rue de l’Ile d’Yeu, Nantes CEDEX 3, France; [email protected] (D.P.); fl[email protected] (F.F.); [email protected] (L.M.); [email protected] (L.K.); [email protected] (S.B.); [email protected] (C.S.); [email protected] (V.V.-B.); [email protected] (S.C.-J.) 2 Ifremer, Bretagne Center, Health, Environment and Microbiology, 1625 Route de Sainte-Anne, 29280 Plouzané, France; [email protected] * Correspondence: [email protected] Received: 17 September 2019; Accepted: 13 November 2019; Published: 19 November 2019 Featured Application: By analyzing extracts of biofilm formed by two marine bacteria and comparing them with planktonic extracts, we have shown that biofilm may induce the biosynthesis of potentially bioactive compounds and may open up new possibilities for compound discovery. Abstract: In the marine environment, biofilm formation is an important lifestyle for microorganisms. A biofilm is comprised of cells embedded in an extracellular matrix that holds them close together and keeps the biofilm attached to the colonized surface. This predominant lifestyle and its main regulation pathway, namely quorum-sensing (QS), have been shown to induce specific bioactive metabolites. In this study, we investigated the biofilm formation by two marine bacteria belonging to the Vibrio species to discover potentially innovative bioactive compounds. -

The Origin of the Eukaryotic Cell Based on Conservation of Existing
The Origin of the Eukaryotic Albert D. G. de Roos The Beagle Armada Cell Based on Conservation Bioinformatics Division of Existing Interfaces Einsteinstraat 67 3316GG Dordrecht, The Netherlands [email protected] Abstract Current theories about the origin of the eukaryotic Keywords cell all assume that during evolution a prokaryotic cell acquired a Evolution, nucleus, eukaryotes, self-assembly, cellular membranes nucleus. Here, it is shown that a scenario in which the nucleus acquired a plasma membrane is inherently less complex because existing interfaces remain intact during evolution. Using this scenario, the evolution to the first eukaryotic cell can be modeled in three steps, based on the self-assembly of cellular membranes by lipid-protein interactions. First, the inclusion of chromosomes in a nuclear membrane is mediated by interactions between laminar proteins and lipid vesicles. Second, the formation of a primitive endoplasmic reticulum, or exomembrane, is induced by the expression of intrinsic membrane proteins. Third, a plasma membrane is formed by fusion of exomembrane vesicles on the cytoskeletal protein scaffold. All three self-assembly processes occur both in vivo and in vitro. This new model provides a gradual Darwinistic evolutionary model of the origins of the eukaryotic cell and suggests an inherent ability of an ancestral, primitive genome to induce its own inclusion in a membrane. 1 Introduction The origin of eukaryotes is one of the major challenges in evolutionary cell biology. No inter- mediates between prokaryotes and eukaryotes have been found, and the steps leading to eukaryotic endomembranes and endoskeleton are poorly understood. There are basically two competing classes of hypotheses: the endosymbiotic and the autogenic. -

Nerve Tissue-Specific Human Glutamate Dehydrogenase That Is Thermolabile and Highly Regulated by ADP , I *P
Fordham University Masthead Logo DigitalResearch@Fordham Chemistry Faculty Publications Chemistry 1997 Nerve tissue-specific umh an glutamate dehydrogenase that is thermolabile and highly regulated by adp / P. Shashidharan, Donald D. Clarke, Naveed Ahmed, Nicholas Moschonas, and Andreas Plaitakis Department of Neurology, Mount Sinai School of Medicine, New York; Department of Chemistry, Fordham University, Bronx New York, USA; and Department of Biology and School of Health Sciences, University of Crete, Crete, Greece P. Shashidharan Mount Sinai School of Medicine. Department of Neurology, [email protected] Donald Dudley Clarke PhD Fordham University, [email protected] Recommended Citation Shashidharan, P.; Clarke, Donald Dudley PhD; Ahmed, Naveed; and Moschonas, Nicholas, "Nerve tissue-specific umh an glutamate dehydrogenase that is thermolabile and highly regulated by adp / P. Shashidharan, Donald D. Clarke, Naveed Ahmed, Nicholas Moschonas, and Andreas Plaitakis Department of Neurology, Mount Sinai School of Medicine, New York; Department of Chemistry, Fordham University, Bronx New York, USA; and Department of Biology and School of Health Sciences, University of Crete, Crete, Greece" (1997). Chemistry Faculty Publications. 13. https://fordham.bepress.com/chem_facultypubs/13 This Article is brought to you for free and open access by the Chemistry at DigitalResearch@Fordham. It has been accepted for inclusion in Chemistry Faculty Publications by an authorized administrator of DigitalResearch@Fordham. For more information, please contact [email protected]. Naveed Ahmed Mount Sinai School of Medicine. Department of Neurology Nicholas Moschonas University of Crete. Department of Biology Follow this and additional works at: https://fordham.bepress.com/chem_facultypubs Part of the Biochemistry Commons Journal of Neurochemistry Lippincott-Raven Publishers, Philadelphia © 1997 International Society for Neurochemistry Nerve Tissue-Specific Human Glutamate Dehydrogenase that Is Thermolabile and Highly Regulated by ADP , I *P. -

Sensitivity of Golgi Matrix Proteins to an ER Exit Block
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central JCBArticle Evidence that the entire Golgi apparatus cycles in interphase HeLa cells: sensitivity of Golgi matrix proteins to an ER exit block Suzanne Miles,1 Heather McManus,1 Kimberly E. Forsten,2 and Brian Storrie1 1Department of Biochemistry and 2Department of Chemical Engineering, Virginia Tech, Blacksburg, VA 24061 e tested whether the entire Golgi apparatus is concentration. Redistribution of GalNAcT2 was more a dynamic structure in interphase mammalian sensitive to low Sar1pdn concentrations than giantin or Wcells by assessing the response of 12 different GM130. Redistribution was most rapid for p27, COPI, and Golgi region proteins to an endoplasmic reticulum (ER) exit p115. Giantin, GM130, and GalNAcT2 relocated with block. The proteins chosen spanned the Golgi apparatus approximately equal kinetics. Distinct ER accumulation and included both Golgi glycosyltransferases and putative could be demonstrated for all integral membrane proteins. matrix proteins. Protein exit from ER was blocked either by ER-accumulated Golgi region proteins were functional. microinjection of a GTP-restricted Sar1p mutant protein in Photobleaching experiments indicated that Golgi-to-ER the presence of a protein synthesis inhibitor, or by plasmid- protein cycling occurred in the absence of any ER exit encoded expression of the same dominant negative Sar1p. block. We conclude that the entire Golgi apparatus is a All Golgi region proteins examined lost juxtanuclear Golgi dynamic structure and suggest that most, if not all, Golgi apparatus–like distribution as scored by conventional and region–integral membrane proteins cycle through ER in confocal fluorescence microscopy in response to an ER interphase cells. -

Center of Biomedical Research Excellence in Matrix Biology: Building Research Infrastructure, Supporting Young Researchers, and Fostering Collaboration
International Journal of Molecular Sciences Editorial Center of Biomedical Research Excellence in Matrix Biology: Building Research Infrastructure, Supporting Young Researchers, and Fostering Collaboration Julia Thom Oxford 1,2,3,* , Ken A. Cornell 1,3,4, Jared J. Romero 1,5 , Diane B. Smith 1,3 , Tracy L. Yarnell 1,3, Rhiannon M. Wood 1,3 , Cheryl L. Jorcyk 1,2, Trevor J. Lujan 1,6, Allan R. Albig 1,2 , Kristen A. Mitchell 1,2, Owen M. McDougal 1,4 , Daniel Fologea 1,7, David Estrada 1,8, Juliette K. Tinker 1,2, Rajesh Nagarajan 1,4, Don L. Warner 1,4, Troy T. Rohn 1,2, Jim Browning 1,9 , Richard S. Beard Jr. 1,3, Lisa R. Warner 1,4 , Brad E. Morrison 1,2, Clare K. Fitzpatrick 1,6, Gunes Uzer 1,6, Laura Bond 1,3, Stephanie M. Frahs 1,3, Cynthia Keller-Peck 1,3, Xinzhu Pu 1,3, Luke G. Woodbury 1,3 and Matthew W. Turner 1,3 1 Center of Biomedical Research Excellence in Matrix Biology, Boise State University, Boise, ID 83725, USA 2 Department of Biological Sciences, Boise State University, Boise, ID 83725, USA 3 Biomolecular Research Center, Boise State University, Boise, ID 83725, USA 4 Department of Chemistry and Biochemistry, Boise State University, Boise, ID 83725, USA 5 Division of Research, Boise State University, Boise, ID 83725, USA 6 Department of Mechanical and Biomedical Engineering, Boise State University, Boise, ID 83725, USA 7 Department of Physics, Boise State University, Boise, ID 83725, USA 8 Micron School of Materials Science and Engineering, Boise State University, Boise, ID 83725, USA 9 Department of Electrical and Computer Engineering, Boise State University, Boise, ID 83725, USA * Correspondence: [email protected]; Tel.: +01-208-426-2395 Received: 9 March 2020; Accepted: 17 March 2020; Published: 20 March 2020 Abstract: The Center of Biomedical Research Excellence in Matrix Biology strives to improve our understanding of extracellular matrix at molecular, cellular, tissue, and organismal levels to generate new knowledge about pathophysiology, normal development, and regenerative medicine. -
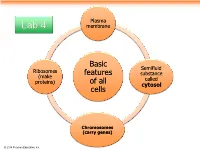
Basic Features of All Cells
Plasma Lab 4 membrane Basic Semifluid Ribosomes features substance (make called proteins) of all cytosol cells Chromosomes (carry genes) © 2014 Pearson Education, Inc. Prokaryotic cells are characterized by having . No nucleus . DNA in an unbound region called the nucleoid . No membrane-bound organelles . Cytoplasm bound by the plasma membrane © 2014 Pearson Education, Inc. Eukaryotic cells are characterized by having • DNA in a nucleus that is bounded by a membranous nuclear envelope • Membrane-bound organelles • Cytoplasm in the region between the plasma membrane and nucleus Eukaryotic cells are generally much larger than prokaryotic cells © 2014 Pearson Education, Inc. Figure 6.8a ENDOPLASMIC RETICULUM (ER) Nuclear envelope Rough ER Smooth ER Nucleolus NUCLEUS Flagellum Chromatin Centrosome Plasma membrane CYTOSKELETON: Microfilaments Intermediate filaments Microtubules Ribosomes Microvilli Golgi apparatus Peroxisome Lysosome Mitochondrion © 2014 Pearson Education, Inc. Figure 6.8b Nuclear envelope NUCLEUS Nucleolus Rough ER Chromatin Smooth ER Ribosomes Golgi Central vacuole apparatus Microfilaments CYTOSKELETON Microtubules Mitochondrion Peroxisome Plasma Chloroplast membrane Cell wall Plasmodesmata Wall of adjacent cell © 2014 Pearson Education, Inc. © 2014 Pearson Education, Inc. © 2014 Pearson Education, Inc. © 2014 Pearson Education, Inc. Table 6.1 © 2014 Pearson Education, Inc. Cell Walls of Plants The cell wall is an extracellular structure that distinguishes plant cells from animal cells Plant cell walls Prokaryotes, are made -

An Endogenous Foamy-Like Viral Element in the Coelacanth Genome
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central An Endogenous Foamy-like Viral Element in the Coelacanth Genome Guan-Zhu Han*, Michael Worobey* Department of Ecology and Evolutionary Biology, University of Arizona, Tucson, Arizona, United States of America Abstract Little is known about the origin and long-term evolutionary mode of retroviruses. Retroviruses can integrate into their hosts’ genomes, providing a molecular fossil record for studying their deep history. Here we report the discovery of an endogenous foamy virus-like element, which we designate ‘coelacanth endogenous foamy-like virus’ (CoeEFV), within the genome of the coelacanth (Latimeria chalumnae). Phylogenetic analyses place CoeEFV basal to all known foamy viruses, strongly suggesting an ancient ocean origin of this major retroviral lineage, which had previously been known to infect only land mammals. The discovery of CoeEFV reveals the presence of foamy-like viruses in species outside the Mammalia. We show that foamy-like viruses have likely codiverged with their vertebrate hosts for more than 407 million years and underwent an evolutionary transition from water to land with their vertebrate hosts. These findings suggest an ancient marine origin of retroviruses and have important implications in understanding foamy virus biology. Citation: Han G-Z, Worobey M (2012) An Endogenous Foamy-like Viral Element in the Coelacanth Genome. PLoS Pathog 8(6): e1002790. doi:10.1371/ journal.ppat.1002790 Editor: Michael Emerman, Fred Hutchinson Cancer Research Center, United States of America Received February 29, 2012; Accepted May 22, 2012; Published June 28, 2012 Copyright: ß 2012 Han, Worobey.