18626-The-Diploic-Veins-A-Comprehensive-Review-With-Clinical-Applications.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Middle Cranial Fossa Sphenoidal Region Dural Arteriovenous Fistulas: Anatomic and Treatment Considerations
ORIGINAL RESEARCH INTERVENTIONAL Middle Cranial Fossa Sphenoidal Region Dural Arteriovenous Fistulas: Anatomic and Treatment Considerations Z.-S. Shi, J. Ziegler, L. Feng, N.R. Gonzalez, S. Tateshima, R. Jahan, N.A. Martin, F. Vin˜uela, and G.R. Duckwiler ABSTRACT BACKGROUND AND PURPOSE: DAVFs rarely involve the sphenoid wings and middle cranial fossa. We characterize the angiographic findings, treatment, and outcome of DAVFs within the sphenoid wings. MATERIALS AND METHODS: We reviewed the clinical and radiologic data of 11 patients with DAVFs within the sphenoid wing that were treated with an endovascular or with a combined endovascular and surgical approach. RESULTS: Nine patients presented with ocular symptoms and 1 patient had a temporal parenchymal hematoma. Angiograms showed that 5 DAVFs were located on the lesser wing of sphenoid bone, whereas the other 6 were on the greater wing of the sphenoid bone. Multiple branches of the ICA and ECA supplied the lesions in 7 patients. Four patients had cortical venous reflux and 7 patients had varices. Eight patients were treated with transarterial embolization using liquid embolic agents, while 3 patients were treated with transvenous embo- lization with coils or in combination with Onyx. Surgical disconnection of the cortical veins was performed in 2 patients with incompletely occluded DAVFs. Anatomic cure was achieved in all patients. Eight patients had angiographic and clinical follow-up and none had recurrence of their lesions. CONCLUSIONS: DAVFs may occur within the dura of the sphenoid wings and may often have a presentation similar to cavernous sinus DAVFs, but because of potential associations with the cerebral venous system, may pose a risk for intracranial hemorrhage. -

Why Should We Report Posterior Fossa Emissary Veins?
Diagn Interv Radiol 2014; 20:78–81 NEURORADIOLOGY © Turkish Society of Radiology 2014 PICTORIAL ESSAY Why should we report posterior fossa emissary veins? Yeliz Pekçevik, Rıdvan Pekçevik ABSTRACT osterior fossa emissary veins pass through cranial apertures and par- Posterior fossa emissary veins are valveless veins that pass ticipate in extracranial venous drainage of the posterior fossa dural through cranial apertures. They participate in extracranial ve- sinuses. These emissary veins are usually small and asymptomatic nous drainage of the posterior fossa dural sinuses. The mas- P toid emissary vein, condylar veins, occipital emissary vein, in healthy people. They protect the brain from increases in intracranial and petrosquamosal sinus are the major posterior fossa emis- pressure in patients with lesions of the neck or skull base and obstructed sary veins. We believe that posterior fossa emissary veins can internal jugular veins (1). They also help to cool venous blood circulat- be detected by radiologists before surgery with a thorough understanding of their anatomy. Describing them using tem- ing through cephalic structures (2). Emissary veins may be enlarged in poral bone computed tomography (CT), CT angiography, patients with high-flow vascular malformations or severe hypoplasia or and cerebral magnetic resonance (MR) venography exam- inations results in more detailed and accurate preoperative aplasia of the jugular veins. They are associated with craniofacial syn- radiological interpretation and has clinical importance. This dromes (1, 3). Dilated emissary veins may cause tinnitus (4, 5). pictorial essay reviews the anatomy of the major and clini- We aim to emphasize the importance of reporting posterior fossa em- cally relevant posterior fossa emissary veins using high-reso- lution CT, CT angiography, and MR venography images and issary veins prior to surgeries that are related to the posterior fossa and discusses the clinical importance of reporting these vascular mastoid region. -

Anatomical Variants of the Emissary Veins: Unilateral Aplasia of Both the Sigmoid Sinus and the Internal Jugular Vein and Development of the Petrosquamosal Sinus
Folia Morphol. Vol. 70, No. 4, pp. 305–308 Copyright © 2011 Via Medica C A S E R E P O R T ISSN 0015–5659 www.fm.viamedica.pl Anatomical variants of the emissary veins: unilateral aplasia of both the sigmoid sinus and the internal jugular vein and development of the petrosquamosal sinus. A rare case report O. Kiritsi1, G. Noussios2, K. Tsitas3, P. Chouridis4, D. Lappas5, K. Natsis6 1“Hippokrates” Diagnostic Centre of Kozani, Greece 2Laboratory of Anatomy in Department of Physical Education and Sports Medicine at Serres, “Aristotle” University of Thessaloniki, Greece 3Orthopaedic Department of General Hospital of Kozani, Greece 4Department of Otorhinolaryngology of “Hippokration” General Hospital of Thessaloniki, Greece 5Department of Anatomy of Medical School of “National and Kapodistrian” University of Athens, Greece 6Department of Anatomy of the Medical School of “Aristotle” University of Thessaloniki, Greece [Received 9 August 2011; Accepted 25 September 2011] We report a case of hypoplasia of the right transverse sinus and aplasia of the ipsilateral sigmoid sinus and the internal jugular vein. In addition, development of the petrosquamosal sinus and the presence of a large middle meningeal sinus and sinus communicans were observed. A 53-year-old Caucasian woman was referred for magnetic resonance imaging (MRI) investigation due to chronic head- ache. On the MRI scan a solitary meningioma was observed. Finally MR 2D veno- graphy revealed this extremely rare variant. (Folia Morphol 2011; 70, 4: 305–308) Key words: hypoplasia, right transverse sinus, aplasia, ipsilateral sigmoid sinus, petrosquamosal sinus, internal jugular vein INTRODUCTION CASE REPORT Emissary veins participate in the extracranial A 53-year-old Caucasian woman was referred for venous drainage of the dural sinuses of the poste- magnetic resonance imaging (MRI) investigation due to rior fossa, complementary to the internal jugular chronic frontal headache complaints. -

CHAPTER 8 Face, Scalp, Skull, Cranial Cavity, and Orbit
228 CHAPTER 8 Face, Scalp, Skull, Cranial Cavity, and Orbit MUSCLES OF FACIAL EXPRESSION Dural Venous Sinuses Not in the Subendocranial Occipitofrontalis Space More About the Epicranial Aponeurosis and the Cerebral Veins Subcutaneous Layer of the Scalp Emissary Veins Orbicularis Oculi CLINICAL SIGNIFICANCE OF EMISSARY VEINS Zygomaticus Major CAVERNOUS SINUS THROMBOSIS Orbicularis Oris Cranial Arachnoid and Pia Mentalis Vertebral Artery Within the Cranial Cavity Buccinator Internal Carotid Artery Within the Cranial Cavity Platysma Circle of Willis The Absence of Veins Accompanying the PAROTID GLAND Intracranial Parts of the Vertebral and Internal Carotid Arteries FACIAL ARTERY THE INTRACRANIAL PORTION OF THE TRANSVERSE FACIAL ARTERY TRIGEMINAL NERVE ( C.N. V) AND FACIAL VEIN MECKEL’S CAVE (CAVUM TRIGEMINALE) FACIAL NERVE ORBITAL CAVITY AND EYE EYELIDS Bony Orbit Conjunctival Sac Extraocular Fat and Fascia Eyelashes Anulus Tendineus and Compartmentalization of The Fibrous "Skeleton" of an Eyelid -- Composed the Superior Orbital Fissure of a Tarsus and an Orbital Septum Periorbita THE SKULL Muscles of the Oculomotor, Trochlear, and Development of the Neurocranium Abducens Somitomeres Cartilaginous Portion of the Neurocranium--the The Lateral, Superior, Inferior, and Medial Recti Cranial Base of the Eye Membranous Portion of the Neurocranium--Sides Superior Oblique and Top of the Braincase Levator Palpebrae Superioris SUTURAL FUSION, BOTH NORMAL AND OTHERWISE Inferior Oblique Development of the Face Actions and Functions of Extraocular Muscles Growth of Two Special Skull Structures--the Levator Palpebrae Superioris Mastoid Process and the Tympanic Bone Movements of the Eyeball Functions of the Recti and Obliques TEETH Ophthalmic Artery Ophthalmic Veins CRANIAL CAVITY Oculomotor Nerve – C.N. III Posterior Cranial Fossa CLINICAL CONSIDERATIONS Middle Cranial Fossa Trochlear Nerve – C.N. -

Torcular Herophili)Ÿ W
Neuroanatomy, 2002, Volume1, Page 14. Letter to the Editor Published online November 7, 2002 © neuroanatomy.org R. Shane Tubbs We would like to clarify a commonly misunderstood term (torcular Herophili)Ÿ W. Jerry Oakes that has infiltrated all fields associated with neuroanatomy e.g. neurosurgery, neurology, neurosciences. The term torcular (wine press) is an incorrect version of the original Greek word (a canal or gutter) [1]. Herophili is after the celebrated Greek physician/anatomist Herophilus (335 B.C.-280 B.C.) born in Chalcedon which is now Kadikoy, Turkey. Herophilus is known as the father of anatomy because he was the first to base his conclusions on dissection of the human body. Herophilus studied the brain, recognizing it as the center Pediatric Neurosurgery, Children’s Hospital, Birmingham, Alabama 35233 USA of the nervous system. The original term was meant to describe the concavity on the internal aspect of the occipital bone that housed the confluence of sinuses. However, over time this term has been used incorrectly as an interchangable term with the confluence of sinuses. Almost every textbook of anatomy with few exceptions, that we reviewed, interchange these terms with no distinction [e.g. 2-4]. True these two entities are intimately related Correspondence Address but clearly represent different anatomical structures. Just as other venous sinuses erode the inner table of the skull producing same named sulci or R. Shane Tubbs, Pediatric Neurosurgery ACC 400, 1600 7th Ave grooves e.g. the transverse sinus sulcus, the confluence of sinuses (formed by South, Birmingham, Alabama 35233 USA the superior sagittal, straight, occipital, and transverse sinuses) erode the Phone: 205-939-9914 Fax: 205-939-9972 occipital bone where the major venous sinus tributaries congregate thus forming E-mail: [email protected] the torcular Herophili. -

Morphology of the Pterion in Serbian Population
Int. J. Morphol., 38(4):820-824, 2020. Morphology of the Pterion in Serbian Population Morfología del Pterion en Población Serbia Knezi Nikola1; Stojsic Dzunja Ljubica1; Adjic Ivan2; Maric Dusica1 & Pupovac Nikolina4 KNEZI, N.; STOJSIC, D. L.; ADJIC, I.; MARIC, D. & PUPOVAC, N. Morphology of the pterion in Serbian population. Int. J. Morphol., 38(4):820-824, 2020. SUMMARY: The pterion is a topographic point on the lateral aspect of the skull where frontal, sphenoid, parietal and temporal bones form the H or K shaped suture. This is an important surgical point for the lesions in anterior and middle cranial fossa. This study was performed on 50 dry skulls from Serbian adult individuals from Department of Anatomy, Faculty of Medicine in Novi Sad. The type of the pterion on both sides of each skull was determined and they are calcified in four types (sphenoparietal, frontotemporal, stellate and epipteric). The distance between the center of the pterion and defined anthropological landmarks were measured using the ImageJ software. Sphenoparietal type is predominant with 86 % in right side and 88 % in left side. In male skulls, the distance from the right pterion to the frontozygomatic suture is 39.89±3.85 mm and 39.67±4.61 mm from the left pterion to the frontozygomatic suture. In female skulls the distance is 37.38±6.38 mm on the right and 35.94±6.46 mm on the left. The shape and the localization of the pterion are important because it is an anatomical landmark and should be used in neurosurgery, traumatology and ophthalmology. -

CLOSURE of CRANIAL ARTICULATIONS in the SKULI1 of the AUSTRALIAN ABORIGINE by A
CLOSURE OF CRANIAL ARTICULATIONS IN THE SKULI1 OF THE AUSTRALIAN ABORIGINE By A. A. ABBIE, Department of Anatomy, University of Adelaide INTRODUCTION While it is well known that joint closure advances more or less progressively with age, there is still little certainty in matters of detail, mainly for lack of adequate series of documented skulls. In consequence, sundry beliefs have arisen which tend to confuse the issue. One view, now disposed of (see Martin, 1928), is that early suture closure indicates a lower or more primitive type of brain. A corollary, due to Broca (see Topinard, 1890), that the more the brain is exercised the more is suture closure postponed, is equally untenable. A very widespread belief is based on Gratiolet's statement (see Topinard, 1890; Frederic, 1906; Martin, 1928; Fenner, 1939; and others) that in 'lower' skulls the sutures are simple and commence to fuse from in front, while in 'higher' skulls the sutures are more complicated and tend to fuse from behind. This view was disproved by Ribbe (quoted from Frederic, 1906), who substituted the generalization that in dolicocephals synostosis begins in the coronal suture, and in brachycephals in the lambdoid suture. In addition to its purely anthropological interest the subject raises important biological considerations of brain-skull relationship, different foetalization in different ethnological groups (see Bolk, 1926; Weidenreich, 1941; Abbie, 1947), and so on. A survey of the literature reveals very little in the way of data on the age incidence of suture closure. The only substantial contribution accessible here comes from Todd & Lyon (1924) for Europeans, but their work is marred by arbitrary rejection of awkward material. -
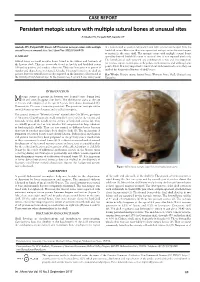
Persistent Metopic Suture with Multiple Sutural Bones at Unusual Sites
CASE REPORT Persistent metopic suture with multiple sutural bones at unusual sites Ambade HV, Fulpatil MP, Kasote AP Ambade HV, Fulpatil MP, Kasote AP. Persistent metopic suture with multiple in a human skull at asterion, left pterion and right coronal suture apart from the sutural bones at unusual sites. Int J Anat Var. 2017;10(3):69-70. lambdoid suture. Moreover, there was a persistent metopic suture between bregma to nasion in the same skull. The metopic suture with multiple sutural bones SUMMARY spreading beyond lambdoid suture at unusual sites is not reported previously. The knowledge of such variation and combination is rare and very important Sutural bones are small irregular bones found in the sutures and fontanels of for forensic expert, radiologists, orthopedists, neurosurgeons and anthropologist the human skull. They are commonly found at lambda and lambdoid suture point of view. It is very important to know about such variation because they can followed by pterion; and rarely at other sites. They vary from person to person in mislead the diagnosis of fracture of skull bones. number and shape, hence not named. Usually, 1-3 sutural bones in one skull are present, but 8-10 sutural bones are also reported in the literature, all restricted in Key Words: Metopic suture; Sutural bones; Wormian bones; Skull; Unusual sites; the vicinity of lambdoid sutures. In the present case, 8 sutural bones were present Variations INTRODUCTION etopic suture is present in between two frontal bones during fetal Mlife and soon disappear after birth. The obliteration starts at the age of 2 years and completed at the age of 8 years from above downwards (1). -

Spontaneous Encephaloceles of the Temporal Lobe
Neurosurg Focus 25 (6):E11, 2008 Spontaneous encephaloceles of the temporal lobe JOSHUA J. WIND , M.D., ANTHONY J. CAPUTY , M.D., AND FABIO ROBE R TI , M.D. Department of Neurological Surgery, George Washington University, Washington, DC Encephaloceles are pathological herniations of brain parenchyma through congenital or acquired osseus-dural defects of the skull base or cranial vault. Although encephaloceles are known as rare conditions, several surgical re- ports and clinical series focusing on spontaneous encephaloceles of the temporal lobe may be found in the otological, maxillofacial, radiological, and neurosurgical literature. A variety of symptoms such as occult or symptomatic CSF fistulas, recurrent meningitis, middle ear effusions or infections, conductive hearing loss, and medically intractable epilepsy have been described in patients harboring spontaneous encephaloceles of middle cranial fossa origin. Both open procedures and endoscopic techniques have been advocated for the treatment of such conditions. The authors discuss the pathogenesis, diagnostic assessment, and therapeutic management of spontaneous temporal lobe encepha- loceles. Although diagnosis and treatment may differ on a case-by-case basis, review of the available literature sug- gests that spontaneous encephaloceles of middle cranial fossa origin are a more common pathology than previously believed. In particular, spontaneous cases of posteroinferior encephaloceles involving the tegmen tympani and the middle ear have been very well described in the medical literature. -

Dural Venous Channels: Hidden in Plain Sight–Reassessment of an Under-Recognized Entity
Published July 16, 2020 as 10.3174/ajnr.A6647 ORIGINAL RESEARCH INTERVENTIONAL Dural Venous Channels: Hidden in Plain Sight–Reassessment of an Under-Recognized Entity M. Shapiro, K. Srivatanakul, E. Raz, M. Litao, E. Nossek, and P.K. Nelson ABSTRACT BACKGROUND AND PURPOSE: Tentorial sinus venous channels within the tentorium cerebelli connecting various cerebellar and su- pratentorial veins, as well as the basal vein, to adjacent venous sinuses are a well-recognized entity. Also well-known are “dural lakes” at the vertex. However, the presence of similar channels in the supratentorial dura, serving as recipients of the Labbe, super- ficial temporal, and lateral and medial parieto-occipital veins, among others, appears to be underappreciated. Also under-recog- nized is the possible role of these channels in the angioarchitecture of certain high-grade dural fistulas. MATERIALS AND METHODS: A retrospective review of 100 consecutive angiographic studies was performed following identification of index cases to gather data on the angiographic and cross-sectional appearance, location, length, and other features. A review of 100 consecutive dural fistulas was also performed to identify those not directly involving a venous sinus. RESULTS: Supratentorial dural venous channels were found in 26% of angiograms. They have the same appearance as those in the tentorium cerebelli, a flattened, ovalized morphology owing to their course between 2 layers of the dura, in contradistinction to a rounded cross-section of cortical and bridging veins. They are best appreciated on angiography and volumetric postcontrast T1- weighted images. Ten dural fistulas not directly involving a venous sinus were identified, 6 tentorium cerebelli and 4 supratentorial. -

Normal Flow Signal of the Pterygoid Plexus on 3T MRA in Patients Without DAVF of the Cavernous Sinus
ORIGINAL RESEARCH EXTRACRANIAL VASCULAR Normal Flow Signal of the Pterygoid Plexus on 3T MRA in Patients without DAVF of the Cavernous Sinus K. Watanabe, S. Kakeda, R. Watanabe, N. Ohnari, and Y. Korogi ABSTRACT BACKGROUND AND PURPOSE: Cavernous sinuses and draining dural sinuses or veins are often visualized on 3D TOF MRA images in patients with dural arteriovenous fistulas involving the CS. Flow signals may be seen in the jugular vein and dural sinuses at the skull base on MRA images in healthy participants, however, because of reverse flow. Our purpose was to investigate the prevalence of flow signals in the pterygoid plexus and CS on 3T MRA images in a cohort of participants without DAVFs. MATERIALS AND METHODS: Two radiologists evaluated the flow signals of the PP and CS on 3T MRA images obtained from 406 consecutive participants by using a 5-point scale. In addition, the findings on 3T MRA images were compared with those on digital subtraction angiography images in an additional 171 participants who underwent both examinations. RESULTS: The radiologists identified 110 participants (27.1%; 108 left, 10 right, 8 bilateral) with evidence of flow signals in the PP alone (n ϭ 67) or in both the PP and CS (n ϭ 43). Flow signals were significantly more common in the left PP than in the right PP. In 171 patients who underwent both MRA and DSA, the MRA images showed flow signals in the PP with or without CS in 60 patients; no DAVFs were identified on DSA in any of these patients. CONCLUSIONS: Flow signals are frequently seen in the left PP on 3T MRA images in healthy participants. -

MBB: Head & Neck Anatomy
MBB: Head & Neck Anatomy Skull Osteology • This is a comprehensive guide of all the skull features you must know by the practical exam. • Many of these structures will be presented multiple times during upcoming labs. • This PowerPoint Handout is the resource you will use during lab when you have access to skulls. Mind, Brain & Behavior 2021 Osteology of the Skull Slide Title Slide Number Slide Title Slide Number Ethmoid Slide 3 Paranasal Sinuses Slide 19 Vomer, Nasal Bone, and Inferior Turbinate (Concha) Slide4 Paranasal Sinus Imaging Slide 20 Lacrimal and Palatine Bones Slide 5 Paranasal Sinus Imaging (Sagittal Section) Slide 21 Zygomatic Bone Slide 6 Skull Sutures Slide 22 Frontal Bone Slide 7 Foramen RevieW Slide 23 Mandible Slide 8 Skull Subdivisions Slide 24 Maxilla Slide 9 Sphenoid Bone Slide 10 Skull Subdivisions: Viscerocranium Slide 25 Temporal Bone Slide 11 Skull Subdivisions: Neurocranium Slide 26 Temporal Bone (Continued) Slide 12 Cranial Base: Cranial Fossae Slide 27 Temporal Bone (Middle Ear Cavity and Facial Canal) Slide 13 Skull Development: Intramembranous vs Endochondral Slide 28 Occipital Bone Slide 14 Ossification Structures/Spaces Formed by More Than One Bone Slide 15 Intramembranous Ossification: Fontanelles Slide 29 Structures/Apertures Formed by More Than One Bone Slide 16 Intramembranous Ossification: Craniosynostosis Slide 30 Nasal Septum Slide 17 Endochondral Ossification Slide 31 Infratemporal Fossa & Pterygopalatine Fossa Slide 18 Achondroplasia and Skull Growth Slide 32 Ethmoid • Cribriform plate/foramina