Proquest Dissertations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synthesis and Kinetics of Novel Ionic Liquid Soluble Hydrogen Atom Transfer Reagents
Synthesis and kinetics of novel ionic liquid soluble hydrogen atom transfer reagents Thomas William Garrard Submitted in total fulfilment of the requirements of the degree Doctor of Philosophy June 2018 School of Chemistry The University of Melbourne Produced on archival quality paper ORCID: 0000-0002-2987-0937 Abstract The use of radical methodologies has been greatly developed in the last 50 years, and in an effort to continue this progress, the reactivity of radical reactions in greener alternative solvents is desired. The work herein describes the synthesis of novel hydrogen atom transfer reagents for use in radical chemistry, along with a comparison of rate constants and Arrhenius parameters. Two tertiary thiol-based hydrogen atom transfer reagents, 3-(6-mercapto-6-methylheptyl)-1,2- dimethyl-3H-imidazolium tetrafluoroborate and 2-methyl-7-(2-methylimidazol-1-yl)heptane-2-thiol, have been synthesised. These are modelled on traditional thiol reagents, with a six-carbon chain with an imidazole ring on one end and tertiary thiol on the other. 3-(6-mercapto-6-methylheptyl)- 1,2-dimethyl-3H-imidazolium tetrafluoroborate comprises of a charged imidazolium ring, while 2- methyl-7-(2-methylimidazol-1-yl)heptane-2-thiol has an uncharged imidazole ring in order to probe the impact of salt formation on radical kinetics. The key step in the synthesis was addition of thioacetic acid across an alkene to generate a tertiary thioester, before deprotection with either LiAlH4 or aqueous NH3. Arrhenius plots were generated to give information on rate constants for H-atom transfer to a primary alkyl radical, the 5-hexenyl radical, in ethylmethylimidazolium bis(trifluoromethane)sulfonimide. -

Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life
Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life Item Type text; Electronic Dissertation Authors Pasek, Matthew Adam Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 07/10/2021 06:16:37 Link to Item http://hdl.handle.net/10150/194288 PHOSPHORUS AND SULFUR COSMOCHEMISTRY: IMPLICATIONS FOR THE ORIGINS OF LIFE by Matthew Adam Pasek ________________________ A Dissertation Submitted to the Faculty of the DEPARTMENT OF PLANETARY SCIENCE In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College UNIVERSITY OF ARIZONA 2 0 0 6 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Matthew Adam Pasek entitled Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy _______________________________________________________________________ Date: 04/11/2006 Dante Lauretta _______________________________________________________________________ Date: 04/11/2006 Timothy Swindle _______________________________________________________________________ Date: 04/11/2006 -

Vanadate-Molybdate Reagent Safety Data Sheet According to Federal Register / Vol
Vanadate-Molybdate Reagent Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 12/20/2013 Revision date: 05/02/2014 Supersedes: 12/20/2013 Version: 1.1 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Mixture Product name : Vanadate-Molybdate Reagent Product code : LC26600 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/mixture : For laboratory and manufacturing use only. 1.3. Details of the supplier of the safety data sheet LabChem Inc Jackson's Pointe Commerce Park Building 1000, 1010 Jackson's Pointe Court Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or 011-703-527-3887 SECTION 2: Hazards identification 2.1. Classification of the substance or mixture GHS-US classification Skin Corr. 1B H314 Eye Dam. 1 H318 2.2. Label elements GHS-US labelling Hazard pictograms (GHS-US) : GHS05 Signal word (GHS-US) : Danger Hazard statements (GHS-US) : H314 - Causes severe skin burns and eye damage Precautionary statements (GHS-US) : P260 - Do not breathe mist, vapours, spray P264 - Wash exposed skin thoroughly after handling P280 - Wear protective gloves, eye protection, protective clothing, face protection P301+P330+P331 - IF SWALLOWED: rinse mouth. Do NOT induce vomiting P303+P361+P353 - IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower P304+P340 - IF INHALED: remove victim to fresh air and keep at rest in a position comfortable for breathing P305+P351+P338 - If in eyes: Rinse cautiously with water for several minutes. -

Differential Induction of Apoptosis in Swiss 3T3 Cells by Nitric Oxide and the Nitrosonium Cation
Journal of Cell Science 110, 2315-2322 (1997) 2315 Printed in Great Britain © The Company of Biologists Limited 1997 JCS4287 Differential induction of apoptosis in Swiss 3T3 cells by nitric oxide and the nitrosonium cation Shazia Khan1,2, Midori Kayahara1,2, Umesh Joashi2, Nicholas D. Mazarakis2, Catherine Sarraf3, A. David Edwards2, Martin N. Hughes1 and Huseyin Mehmet2,* 1Department of Chemistry, King’s College London, Strand, London WC2R 2LS, UK 2Weston Laboratory, Department of Paediatrics and Neonatal Medicine, and 3Department of Histopathology, Royal Postgraduate Medical School, Hammersmith Hospital, Du Cane Road, London W12 0NN, UK *Author for correspondence (e-mail: [email protected]) SUMMARY We have investigated the effect of nitric oxide (NO) on decomposition to NO. The apoptotic effect of NO was apoptosis in Swiss 3T3 fibroblasts and compared it to the reduced in the presence of the NO scavenger oxyhaemo- effect of the nitrosonium cation (NO+). Both species globin, or the antioxidants N-acetylcysteine and ascorbic induced apoptosis, confirmed by electron microscopy, acid, whereas in the case of NO+ these antioxidants poten- propidium iodide staining, DNA laddering and activation tiated apoptosis. Glutathione also had a potentiating effect of caspases. The kinetics of triggering apoptosis were on the cytotoxicity of NO+. This suggests that cellular different for the two redox species: NO+ required only a 2 antioxidants may play a role in protecting the cell from NO- hour exposure, whereas NO required 24 hours. Three induced apoptosis while NO+ may trigger apoptosis inde- sources of NO were used: aqueous solutions of NO and two pendently of oxidative stress mechanisms. -

Nitric Oxide Releasing Chelating Agents and Their
Europäisches Patentamt *EP001060174B1* (19) European Patent Office Office européen des brevets (11) EP 1 060 174 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.7: C07D 401/12, A61K 31/44 of the grant of the patent: 22.09.2004 Bulletin 2004/39 (86) International application number: PCT/GB1998/003840 (21) Application number: 98962567.8 (87) International publication number: (22) Date of filing: 18.12.1998 WO 1999/033823 (08.07.1999 Gazette 1999/27) (54) NITRIC OXIDE RELEASING CHELATING AGENTS AND THEIR THERAPEUTIC USE STICKSTOFFOXID FREISETZENDE CHELATBILDNER UND IHRE THERAPEUTISCHE VERWENDUNG CHELATEURS LIBERANT DE L’OXYDE NITRIQUE ET LEUR EMPLOI A DES FINS THERAPEUTIQUES (84) Designated Contracting States: (74) Representative: AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU Hammett, Audrey Grace Campbell et al MC NL PT SE Amersham plc Amersham Place (30) Priority: 23.12.1997 GB 9727226 Little Chalfont, Bucks. HP7 9NA (GB) 13.03.1998 GB 9805450 (56) References cited: (43) Date of publication of application: EP-A- 0 292 761 WO-A-93/20806 20.12.2000 Bulletin 2000/51 WO-A-95/12394 WO-A-96/31217 WO-A-96/39409 WO-A-97/49390 (73) Proprietor: Amersham Health AS US-A- 5 250 550 0401 Oslo (NO) • MOORADIAN D L ET AL: "NITRIC OXIDE (NO) (72) Inventors: DONOR MOLECULES: EFFECT OF NO • TOWART, Robertson RELEASE RATE ON VASCULAR SMOOTH Stoke Poges SL2 4PT (GB) MUSCLE CELL PROLIFERATION IN VITRO" • KARLSSON, Jan, Olof, Gustav JOURNAL OF CARDIOVASCULAR N-1450 Nesoddtangen (NO) PHARMACOLOGY, vol. -

Oxidation State Slides.Key
Oxidation States Dr. Sobers’ Lecture Slides The Oxidation State Also known as the oxidation number The oxidation state is used to determine whether an element has been oxidized or reduced. The oxidation state is not always a real, quantitative, physical constant. The oxidation state can be the charge on an atom: 2+ - MgCl2 Mg Cl Oxidation State: +2 -1 2 The Oxidation State For covalently bonded substances, it is not as simple as an ionic charge. A covalent bond is a sharing of electrons. The electrons are associated with more than one atomic nuclei. This holds the nuclei together. The electrons may not be equally shared. This creates a polar bond. The electronegativity of a covalently bonded atom is its ability to attract electrons towards itself. 3 Example: Chlorine Sodium chloride is an ionic compound. In sodium chloride, the chloride ion has a charge and an oxidation state of -1. The oxidation state of sodium is +1. 4 Example: Chlorine In a chlorine molecule, the chlorine atoms are covalently bonded. The two atoms share electrons equally and the oxidation state is 0. 5 Example: Chlorine The two atoms of a hydrogen chloride molecule are covalently bonded. The electrons are not shared equally because chlorine is more electronegative than hydrogen. There are no ions but the oxidation state of chlorine in HCl is -1 and the oxidation state of hydrogen is +1. 6 7 Assigning Oxidation States See the handout for the list of rules. Rule 1: Free Elements Free elements have an oxidation state of zero Example Oxidation State O2(g) 0 Fe(s) 0 O3(g) 0 C(graphite) 0 C(diamond) 0 9 Rule 2: Monatomic Ions The oxidation state of monatomic ions is the charge of the ion Example Oxidation State O2- -2 Fe3+ +3 Na+ +1 I- -1 V4+ +4 10 Rule 3: Fluorine in Compounds Fluorine in a compound always has an oxidation state of -1 Example Comments and Oxidation States NaF These are monatomic ions. -

Mechanisms of Nitric Oxide Reactions Mediated by Biologically Relevant Metal Centers
Struct Bond (2014) 154: 99–136 DOI: 10.1007/430_2013_117 # Springer-Verlag Berlin Heidelberg 2013 Published online: 5 October 2013 Mechanisms of Nitric Oxide Reactions Mediated by Biologically Relevant Metal Centers Peter C. Ford, Jose Clayston Melo Pereira, and Katrina M. Miranda Abstract Here, we present an overview of mechanisms relevant to the formation and several key reactions of nitric oxide (nitrogen monoxide) complexes with biologically relevant metal centers. The focus will be largely on iron and copper complexes. We will discuss the applications of both thermal and photochemical methodologies for investigating such reactions quantitatively. Keywords Copper Á Heme models Á Hemes Á Iron Á Metalloproteins Á Nitric oxide Contents 1 Introduction .................................................................................. 101 2 Metal-Nitrosyl Bonding ..................................................................... 101 3 How Does the Coordinated Nitrosyl Affect the Metal Center? .. .. .. .. .. .. .. .. .. .. .. 104 4 The Formation and Decay of Metal Nitrosyls ............................................. 107 4.1 Some General Considerations ........................................................ 107 4.2 Rates of NO Reactions with Hemes and Heme Models ............................. 110 4.3 Mechanistic Studies of NO “On” and “Off” Reactions with Hemes and Heme Models ................................................................................. 115 4.4 Non-Heme Iron Complexes .......................................................... -

Structure-Property Relationships of Layered Oxypnictides
AN ABSTRACT OF THE DISSERTATION OF Sean W. Muir for the degree of Doctor of Philosophy in Chemistry presented on April 17, 2012. Title: Structure-property Relationships of Layered Oxypnictides Abstract approved:______________________________________________________ M. A. Subramanian Investigating the structure-property relationships of solid state materials can help improve many of the materials we use each day in life. It can also lead to the discovery of materials with interesting and unforeseen properties. In this work the structure property relationships of newly discovered layered oxypnictide phases are presented and discussed. There has generally been worldwide interest in layered oxypnictide materials following the discovery of superconductivity up to 55 K for iron arsenides such as LnFeAsO1-xFx (where Ln = Lanthanoid). This work presents efforts to understand the structure and physical property changes which occur to LnFeAsO materials when Fe is replaced with Rh or Ir and when As is replaced with Sb. As part of this work the solid solution between LaFeAsO and LaRhAsO was examined and superconductivity is observed for low Rh content with a maximum critical temperature of 16 K. LnRhAsO and LnIrAsO compositions are found to be metallic; however Ce based compositions display a resistivity temperature dependence which is typical of Kondo lattice materials. At low temperatures a sudden drop in resistivity occurs for both CeRhAsO and CeIrAsO compositions and this drop coincides with an antiferromagnetic transition. The Kondo scattering temperatures and magnetic transition temperatures observed for these materials can be rationalized by considering the expected difference in N(EF)J parameters between them, where N(EF) is the density of states at the Fermi level and J represents the exchange interaction between the Ce 4f1 electrons and the conduction electrons. -

Vanadate and Peroxovanadate Complexes of Biomedical Relevance – a Speciation Approach with Focus on Diabetes
Vanadate and Peroxovanadate Complexes of Biomedical Relevance – A speciation approach with focus on diabetes András Gorzsás Department of Chemistry Inorganic Chemistry Umeå University Umeå, Sweden Akademisk avhandling som med tillstånd av rektorsämbetet vid Umeå Universitet för erhållande av Filosofie Doktorsexamen framlägges till offentlig granskning vid Kemiska institutionen, sal KB3A9, KBC–huset, fredagen den 22 april 2005, kl 13.00. Fakultetsopponent: Professor Carlos F. G. C. Geraldes, Department of Biochemistry, Faculty of Science and Technology, University of Coimbra, P. O. Box 3126, P – 3001 – 401 Coimbra, Portugal i TITLE Vanadate and Peroxovanadate Complexes of Biomedical Relevance – A speciation approach with focus on diabetes AUTHOR András Gorzsás ADDRESS Department of Chemistry, Inorganic Chemistry, Umeå University, SE – 901 87 Umeå, Sweden ABSTRACT Diabetes mellitus is one of the most threatening epidemics of modern times with rapidly increasing incidence. Vanadium and peroxovanadium compounds have been shown to exert insulin–like actions and, in contrast to insulin, are orally applicable. However, problems with side–effects and toxicity remain. The exact mechanism(s) by which these compounds act are not yet fully known. Thus, a better understanding of the aqueous chemistry of vanadates and peroxovanadates in the presence of various (bio)ligands is needed. The present thesis summarises six papers dealing mainly with aqueous speciation in different vanadate – and peroxovanadate – ligand systems of biological and medical relevance. Altogether, five ligands have been studied, including important blood constituents (lactate, citrate and phosphate), a potential drug candidate (picolinic acid), and a dipeptide (alanyl serine) to model the interaction of (peroxo)vanadate in the active site of enzymes. Since all five ligands have been studied both with vanadates and peroxovanadates, the number of systems described in the present work is eleven, including the vanadate – citrate – lactate mixed ligand system. -

List of Reactive Chemicals
LIST OF REACTIVE CHEMICALS Chemical Prefix Chemical Name Reactive Reactive Reactive CAS# Chemical Chemical Chemical Stimulus 1 Stimulus 2 Stimulus 3 111-90-0 "CARBITOL" SOLVENT D 111-15-9 "CELLOSOLVE" ACETATE D 110-80-5 "CELLOSOLVE" SOLVENT D 2- (2,4,6-TRINITROPHENYL)ETHYL ACETATE (1% IN ACETONE & BENZENE S 12427-38-2 AAMANGAN W 88-85-7 AATOX S 40487-42-1 AC 92553 S 105-57-7 ACETAL D 75-07-0 ACETALDEHYDE D 105-57-7 ACETALDEHYDE, DIETHYL ACETAL D 108-05-4 ACETIC ACID ETHENYL ESTER D 108-05-4 ACETIC ACID VINYL ESTER D 75-07-0 ACETIC ALDEHYDE D 101-25-7 ACETO DNPT T 126-84-1 ACETONE DIETHYL ACETAL D 108-05-4 ACETOXYETHYLENE D 108-05-4 1- ACETOXYETHYLENE D 37187-22-7 ACETYL ACETONE PEROXIDE, <=32% AS A PASTE T 37187-22-7 ACETYL ACETONE PEROXIDE, <=42% T 37187-22-7 ACETYL ACETONE PEROXIDE, >42% T S 644-31-5 ACETYL BENZOYL PEROXIDE (SOLID OR MORE THAN 45% IN SOLUTION) T S 644-31-5 ACETYL BENZOYL PEROXIDE, <=45% T 506-96-7 ACETYL BROMIDE W 75-36-5 ACETYL CHLORIDE W ACETYL CYCLOHEXANE SULFONYL PEROXIDE (>82% WITH <12% WATER) T S 3179-56-4 ACETYL CYCLOHEXANE SULFONYL PEROXIDE, <=32% T 3179-56-4 ACETYL CYCLOHEXANE SULFONYL PEROXIDE, <=82% T 674-82-8 ACETYL KETENE (POISON INHALATION HAZARD) D 110-22-5 ACETYL PEROXIDE, <=27% T 110-22-5 ACETYL PEROXIDE, SOLID, OR MORE THAN 27% IN SOLUTION T S 927-86-6 ACETYLCHOLINE PERCHLORATE O S 74-86-2 ACETYLENE D 74-86-2 ACETYLENE (LIQUID) D ACETYLENE SILVER NITRATE D 107-02-08 ACRALDEHYDE (POISON INHALATION HAZARD) D 79-10-7 ACROLEIC ACID D 107-02-08 ACROLEIN, INHIBITED (POISON INHALATION HAZARD) D 107-02-08 ACRYLALDEHYDE (POISON INHALATION HAZARD) D 79-10-7 ACRYLIC ACID D 141-32-2 ACRYLIC ACID BUTYL ESTER D 140-88-5 ACRYLIC ACID ETHYL ESTER D 96-33-3 ACRYLIC ACID METHYL ESTER D Stimulus - Stimuli is the thermal, physical or chemical input needed to induce a hazardous reaction. -
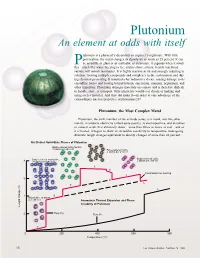
Plutonium Is a Physicist's Dream but an Engineer's Nightmare. with Little
Plutonium An element at odds with itself lutonium is a physicist’s dream but an engineer’s nightmare. With little provocation, the metal changes its density by as much as 25 percent. It can Pbe as brittle as glass or as malleable as aluminum; it expands when it solidi- fies—much like water freezing to ice; and its shiny, silvery, freshly machined surface will tarnish in minutes. It is highly reactive in air and strongly reducing in solution, forming multiple compounds and complexes in the environment and dur- ing chemical processing. It transmutes by radioactive decay, causing damage to its crystalline lattice and leaving behind helium, americium, uranium, neptunium, and other impurities. Plutonium damages materials on contact and is therefore difficult to handle, store, or transport. Only physicists would ever dream of making and using such a material. And they did make it—in order to take advantage of the extraordinary nuclear properties of plutonium-239. Plutonium, the Most Complex Metal Plutonium, the sixth member of the actinide series, is a metal, and like other metals, it conducts electricity (albeit quite poorly), is electropositive, and dissolves in mineral acids. It is extremely dense—more than twice as dense as iron—and as it is heated, it begins to show its incredible sensitivity to temperature, undergoing dramatic length changes equivalent to density changes of more than 20 percent. Six Distinct Solid-State Phases of Plutonium Body-centered orthorhombic, 8 atoms per unit cell Face-centered cubic, 4 atoms per unit cell Body-centered monoclinic, Body-centered cubic, 8 34 atoms per unit cell 2 atoms per unit cell δ δ′ ε Contraction on melting 6 γ L L 4 β Monoclinic, 16 atoms per unit cell Anomalous Thermal Expansion and Phase Instability of Plutonium Length change (%) 2 Pure Pu Pure Al α 0 200 400 600 800 Temperature (°C) 16 Los Alamos Science Number 26 2000 Plutonium Overview Table I. -

HO2 Hydroperoxyl Radical
Railsback's Some Fundamentals of Mineralogy and Geochemistry The multiple forms of oxygen Fully-reduced Not-fully-reduced oxygen; oxygen unstable at some time scale Mineralogists think of oxygen in Nominal its 2- oxidation state in minerals, oxidation and geochemists additionally think number -2 -1 0 about it as elemental diatomic Name of Elemental oxygen (O2) in redox geochemistry. Oxide However, that's just a beginning if state oxygen one additionally considers chemical processes in the atmosphere. As Oxide minerals Diatomic the table at right shows with its blue e.g., MgO oxygen region, atmospheric chemistry additionally deals with ozone, with Oxysalt minerals peroxide, and with oxide gases. O2 The interaction of the less-reduced e.g., CaCO3 Hydroxyl forms of oxygen with the not-fully- & MgFeSiO4 radical Ozone oxidized gases (SO2, NO, NO2, Examples 0 etc.) provides much entertainment Hydroxide minerals OH O3 in atmospheric chemistry. e.g., Al(OH) 3 Hydrogen Atomic One very important point to note here is the difference between OH, ology Hydroxide ion peroxide oxygen the highly reactive hydroxyl radical, Minera - and the hydroxide ion OH-, the OH H2O2 O entity found in water that we can Hydroperoxyl Significant think of as dissociated H2O. In the Water & its vapor in the upper table at right, the OH representing radical atmosphere the peroxide radical has a "0" H2O (>85 km) superscript to remind readers of its HO2 overall neutral charge, but most Aqueous/redox Oxide gases One O0 & authors and printers leave it simply geochemistry e.g., CO, CO , SO one O- as an unadorned "OH".