Array-Based Genomic and Epigenomic Studies in Healthy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
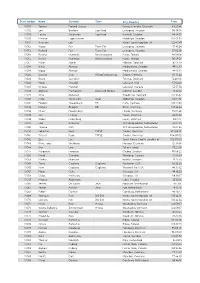
Start Number Name Surname Team City, Country Time 11001 Tommy
Start number Name Surname Team City, Country Time 11001 Tommy Frølund Jensen Kongens lyngby, Denmark 89:20:46 11002 Emil Boström Lag Nord Linköping, Sweden 98:19:04 11003 Lovisa Johansson Lag Nord Burträsk, Sweden 98:19:02 11005 Maurice Lagershausen Huddinge, Sweden 102:01:54 11011 Hyun jun Lee Korea, (south) republic of 122:40:49 11012 Viggo Fält Team Fält Linköping, Sweden 57:45:24 11013 Richard Fält Team Fält Linköping, Sweden 57:45:28 11014 Rasmus Nummela Rackmannarna Pjelax, Finland 98:59:06 11015 Rafael Nummela Rackmannarna Pjelax, Finland 98:59:06 11017 Marie Zølner Hillerød, Denmark 82:11:49 11018 Niklas Åkesson Mellbystrand, Sweden 99:45:22 11019 Viggo Åkesson Mellbystrand, Sweden 99:45:22 11020 Damian Sulik #StopComplaining Siegen, Germany 101:13:24 11023 Bruno Svendsen Tylstrup, Denmark 74:48:08 11024 Philip Oswald Lakeland, USA 55:52:02 11025 Virginia Marshall Lakefield, Canada 55:57:54 11026 Andreas Mathiasson Backyard Heroes Ödsmål, Sweden 75:12:02 11028 Stine Andersen Brædstrup, Denmark 104:35:37 11030 Patte Johansson Tiger Södertälje, Sweden 80:24:51 11031 Frederic Wiesenbach BB Dahn, Germany 101:21:50 11032 Romea Brugger BB Berlin, Germany 101:22:44 11034 Oliver Freudenberg Heyda, Germany 75:07:48 11035 Jan Hennig Farum, Denmark 82:11:50 11036 Robert Falkenberg Farum, Denmark 82:11:51 11037 Edo Boorsma Sint annaparochie, Netherlands 123:17:34 11038 Trijneke Stuit Sint annaparochie, Netherlands 123:17:35 11040 Sebastian Keck TATSE Gieäen, Germany 104:08:01 11041 Tatjana Kage TATSE Gieäen, Germany 104:08:02 11042 Eun Lee -

Startlist IRONMAN 70.3 Westfriesland
Startlist IRONMAN 70.3 Westfriesland Last update: January 14th, 2020 ordered by age group and last name First name Surname Gender Agegroup Country Represented Farida Batenburg FEMALE F18-24 NLD (Netherlands) Morgane Baute FEMALE F18-24 BEL (Belgium) Johanna Geise FEMALE F18-24 DEU (Germany) Odette Helsloot FEMALE F18-24 NLD (Netherlands) Fabienne HoFmann FEMALE F18-24 DEU (Germany) Cindy LeFebvre FEMALE F18-24 BEL (Belgium) Sarah RoeloFs FEMALE F18-24 NLD (Netherlands) MinKe SchurinK FEMALE F18-24 NLD (Netherlands) FemKe van de Water FEMALE F18-24 NLD (Netherlands) Amé Venter FEMALE F18-24 ZAF (South AFrica) Mara Winden FEMALE F18-24 DEU (Germany) Brenda Zwarthoed FEMALE F18-24 NLD (Netherlands) Manar Ali FEMALE F25-29 EGY (Egypt) Jule Bartsch FEMALE F25-29 DEU (Germany) Aurelie Bastijns FEMALE F25-29 BEL (Belgium) Ann-Cathrin Bentrup FEMALE F25-29 DEU (Germany) Mireille BeuKers FEMALE F25-29 NLD (Netherlands) Kristel Bosch FEMALE F25-29 NLD (Netherlands) Sanne Coenen FEMALE F25-29 NLD (Netherlands) NienKe Coenen FEMALE F25-29 NLD (Netherlands) Lejla Crnica FEMALE F25-29 BIH (Bosnia and Herzegovina) Loes Daemen FEMALE F25-29 BEL (Belgium) Ellen de Boer FEMALE F25-29 NLD (Netherlands) Charline DE GREEF FEMALE F25-29 BEL (Belgium) Suuz de Leeuw FEMALE F25-29 NLD (Netherlands) Jessica De Visser FEMALE F25-29 NLD (Netherlands) Justine Debret FEMALE F25-29 FRA (France) 1 Startlist IRONMAN 70.3 Westfriesland Last update: January 14th, 2020 ordered by age group and last name Iris DijKstra FEMALE F25-29 NLD (Netherlands) Charline DUBOS FEMALE -

PHYSICAL THERAPY PRACTICE the Publication of the Academy of Orthopaedic Physical Therapy, APTA
2019 / volume 31 / number 2 ORTHOPAEDIC PHYSICAL THERAPY PRACTICE The publication of the Academy of Orthopaedic Physical Therapy, APTA FEATURE: Effectiveness of a Standing Workstation on Perceived Pain & Function: A Case Series 7239_OP_Apr.indd 501 3/27/19 1:01 PM 7239_OP_Apr.indd 502 3/27/19 1:01 PM Need Help to Prepare for the OCS? Check out AOPT’s Current Concepts & Clinical Practice Guidelines (CPGs) CURRENT CONCEPTS OF ORTHOPAEDIC PHYSICAL THERAPY, 4TH ED. ISC 26.2 Topics and Authors • Clinical Reasoning and Evidence-based Practice— Nicole Christensen, PT, PhD, MAppSc; Benjamin Boyd, PT, DPTSc, OCS; Jason Tonley, PT, DPT, OCS • The Shoulder: Physical Therapy Patient Management Using Current Evidence—Todd S. Ellenbecker, DPT, MS, SCS, OCS, CSCS; Robert C. Manske, DPT, MEd, SCS, ATC, CSCS; Marty Kelley, PT, DPT, OCS • The Elbow: Physical Therapy Patient Management Using Current Evidence—Chris A. Sebelski, PT, DPT, PhD, OCS, CSCS • The Wrist and Hand: Physical Therapy Patient Management Using Current Evidence— Mia Erickson, PT, EdD, CHT, ATC; Carol Waggy, PT, PhD, CHT; Elaine F. Barch, PT, DPT, CHT • The Temporomandibular Joint: Physical Therapy Patient Management Using Current Evidence—Sally Ho, PT, DPT, MS, OCS CURRENT CONCEPTS OF • The Cervical Spine: Physical Therapy Patient Management ORTHOPAEDIC PHYSICAL THERAPY (4th Edition) Using Current Evidence—Michael B. Miller, PT, DPT, OCS, PHYSICAL THERAPY (4th Edition) ISC 26.2, CURRENT CONCEPTS OF ORTHOPAEDIC Independent Study Course 26.2 FAAOMPT, CCI • The Thoracic Spine: Physical Therapy Patient Management Using Current Evidence— Scott Burns, PT, DPT, OCS, FAAOMPT; William Egan, PT, DPT, OCS, FAAOMPT • The Lumbar Spine: Physical Therapy Patient Management Using Current Evidence—Paul F. -
1056 T CELLS and AGING, JANUARY 2002 UPDATE Graham
[Frontiers in Bioscience 7, d1056-1183, May 1, 2002] T CELLS AND AGING, JANUARY 2002 UPDATE Graham Pawelec1, Yvonne Barnett2, Ros Forsey3, Daniela Frasca4, Amiela Globerson5, Julie McLeod6, Calogero Caruso7, Claudio Franceschi8, Támás Fülöp9, Sudhir Gupta10, Erminia Mariani8, Eugenio Mocchegiani11, Rafael Solana12 1University of Tübingen, Center for Medical Research, ZMF, Waldhörnlestr. 22, D-72072 Tübingen, Germany, 2 University of Ulster, Coleraine, UK, 3 Unilever Research, Bedford, UK, 4 University of Miami, FL, 5 University of the Negev, Israel, 6 University of the West of England, Bristol, UK, 7 University of Palermo, Italy, 8 University of Bologna, Italy, 9 University of Sherbrooke, Quebec, Canada, 10 University of California, Irvine, CA, 11 INRCA, Ancona, Italy, 12 University of Córdoba, Spain TABLE OF CONTENTS 1. Abstract 2. Introduction: How do we establish whether immunosenescence exists and if so whether it means anything to immunological defence mechanisms in aging? 3. Factors contributing to immunosenescence 3.1. Hematopoiesis 3.2. Thymus 4. Post-thymic aging 4.1. T cell subsets 4.2. T cell repertoire 4.3. T cell function 4.3.1. Accessory cells 4.3.2. T cell receptor signal transduction 4.3.3. Costimulatory pathways 4.3.3.1. CD28 family coreceptors and CD80 family ligands 4.3.3.2. Other costimulators 4.4. Cytokine production and response 4.4.1. Regulation of gene transcription 4.4.2. Cytokine secretion 4.4.3. Confounding factors affecting cytokine secretion results 4.4.4. Levels of cytokines in plasma 4.4.5. Cytokine antagonists 4.4.6. Cytokine receptor expression 5. Clonal expansion after T cell activation 5.1. -
Bruce Spiegelman Editor
Research and Perspectives in Endocrine Interactions Bruce Spiegelman Editor Hormones, Metabolism and the Benefits of Exercise Research and Perspectives in Endocrine Interactions More information about this series at http://www.springer.com/series/5241 Bruce Spiegelman Editor Hormones, Metabolism and the Benefits of Exercise Editor Bruce Spiegelman Fondation IPSEN Dana-Farber Cancer Institute Boulogne-Billancourt Harvard Medical School France Boston, MA USA Acknowledgement: The editors wish to express their gratitude to Mrs. Mary Lynn Gage for her editorial assistance. ISSN 1861-2253 ISSN 1863-0685 (electronic) Research and Perspectives in Endocrine Interactions ISBN 978-3-319-72789-9 ISBN 978-3-319-72790-5 (eBook) https://doi.org/10.1007/978-3-319-72790-5 Library of Congress Control Number: 2018934440 © The Editor(s) (if applicable) and The Author(s) 2017. This book is an open access publication. Open Access This book is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this book are included in the book’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the book's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. -

Start List As on 7 February 2020 Age Grp Name Surname
START LIST AS ON 7 FEBRUARY 2020 AGE GRP NAME SURNAME AWA CLUB M18-24 Gregor Andrew M18-24 Calvin Andrew M18-24 Marc Andrews M18-24 Lardus Basson M18-24 Henk Blignaut M18-24 Emile Bourreau-Nel My Training M18-24 Francois Burden Day M18-24 Neil De Kock M18-24 Gareth D'Enis-Rowson M18-24 Tyron Dolloway M18-24 Andries Du Toit M18-24 Logan Duffy M18-24 Adrian Erasmus AWA My Training M18-24 Michael Ferreira Bronze Day M18-24 Richard Friend M18-24 Cameron Grassi M18-24 Hugo Grotius M18-24 Ivan Harkema M18-24 Wynand Hugo M18-24 Berndt Jacobs M18-24 Luke Joseph M18-24 Luca La Monica M18-24 Kyle Lello M18-24 Matthew Little M18-24 Robert Mason M18-24 Konrad Meissner-Roloff M18-24 Gerhard Naude M18-24 Jean Naude M18-24 Nicholas Oberholzer M18-24 Pierre Pienaar M18-24 Hermanus Potgieter M18-24 Jeandre Radyn M18-24 Christian Rezek M18-24 Hayden Sheehan M18-24 Shane Sheehan M18-24 JJ Smit M18-24 Gerbrand Smit M18-24 Strauss Van Der Westhuizen M18-24 Frederik Van Dyk M18-24 Michael Walsh AWA Wingman M18-24 Jordan Williams Bronze Multisport START LIST AS ON 7 FEBRUARY 2020 M18-24 Jake Zilesnick M25-29 Nicki Abel M25-29 Ernst Ackermann M25-29 Guy Alexander M25-29 Rob Anderson Trifactri M25-29 Graeme Andrews M25-29 Gareth Angus M25-29 Phivo Artemides M25-29 Josh Ash M25-29 Jarryd Bates M25-29 Matthew Bates M25-29 Gabriel Bayhack M25-29 Altus Beeston M25-29 Manuel Benitez M25-29 Oelof Bergh M25-29 Johan Bester M25-29 Andrew Blane M25-29 Stuart Bleloch AWA M25-29 Japie Blignaut Bronze M25-29 Louis Boshoff AWA M25-29 E'Duan Bosman Bronze M25-29 Markus Botes -

The Effects of Servant Leadership on Follower Performance and Well-Being
Some pages of this thesis may have been removed for copyright restrictions. If you have discovered material in Aston Research Explorer which is unlawful e.g. breaches copyright, (either yours or that of a third party) or any other law, including but not limited to those relating to patent, trademark, confidentiality, data protection, obscenity, defamation, libel, then please read our Takedown policy and contact the service immediately ([email protected]) THE EFFECTS OF SERVANT LEADERSHIP ON FOLLOWER PERFORMANCE AND WELL-BEING: UNDERLYING MECHANISMS, BOUNDARY CONDITIONS, AND THE ROLE OF TRAINING SVEN LOHREY Doctor of Philosophy ASTON UNIVERSITY September 2015 © Sven Lohrey, 2015. Sven Lohrey asserts his moral right to be identified as the author of this thesis. This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognize that its copyright rests with the author and that no quotation from the thesis and no information derived from it may be published without appropriate permission or acknowledge- ment. 1 ASTON UNIVERSITY THE EFFECTS OF SERVANT LEADERSHIP ON FOLLOWER PERFORMANCE AND WELL-BEING: UNDERLYING MECHANISMS, BOUNDARY CONDITIONS, AND THE ROLE OF TRAINING Sven Lohrey Doctor of Philosophy 2015 THESIS SUMMARY Based on a review of the servant leadership, well-being, and performance literatures, the first study develops a research model that examines how and under which conditions servant leadership is related to follower performance and well-being alike. Data was collected from 33 -

South African Human Sciences Research Networking Directory
DOCUMENT RESUME ED 423 185 SO 028 693 AUTHOR van der Berg, Henda, Ed.; Maree-Snijders, Asa, Ed.; Prinsloo, Roelf, Ed. TITLE South African Human Sciences Research Networking Directory. First Edition. INSTITUTION Human Sciences Research Council, Pretoria (South Africa). ISBN ISBN-0-7969-1725-6 PUB DATE 1996-00-00 NOTE 528p.; For related directory, see SO 028 692. PUB TYPE Reference Materials - Directories/Catalogs (132) EDRS PRICE MF02/PC22 Plus Postage. DESCRIPTORS Development; Economic Development; International Cooperation; International Organizations; National Organizations; Organizations (Groups); *Professional Associations; *Research Opportunities IDENTIFIERS South Africa ABSTRACT This networking directory is a comprehensive reference source of names, locations, and fields of interest of South Africanhuman sciences researchers. The guide is intended to promote research cooperation, facilitate networking, and organize conferences. The directory is intended for use at both the international level and the local level. The guide is divided into two sections. Part One offers "Biographical Profiles A to Z," and Part Two a "Subject index." This guide is designed to be used in conjunction with the "Directory of Human Sciences Research Organizations and Professional Associations in South Africa" published in 1995. (EH) ******************************************************************************** Reproductions supplied by EDRS are the best that can be made from the original document. ******************************************************************************** Sc)' FIRST EDITION 1996 SOUTH AFRICAN HUMAN SCIENCES RESEARCH NETWORKING mc DIRECTORY U.S. DEPARTMENT OF EDUCATION PERMISSION TO REPRODUCE AND Office of Educational Research and Improvement DISSEMINATE THIS MATERIAL HAS iED CATIONAL RESOURCES INFORMATION BEEN GRANTED BY CENTER (ERIC) This document has been reproduced as received from the person or organization m er originating it. 0 Minor changes have been made to improve reproduction quality.