A New Species of Drosera Section Arachnopus (Droseraceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Documentary Records Locating Aboriginal People on Mulga Downs Pre 1922 Identified Marriage / Death Certificates (From W.A
Documentary Records locating Aboriginal people on Mulga Downs pre 1922 Identified Marriage / Death certificates (from W.A. Registry Services) - Death Certificate Registration 18/1955, Roebourne district - Tommy Tucker (stockman) male aged 73 born (c.1882) at Mulga Downs Station, son of unknown parents, no marriage details recorded, no children recorded, deceased 10/12/1955 at Roebourne district hospital, buried 10/12/1955 in native portion Roebourne cemetery, last resident at Mulga Downs station prior to death. Note: witnesses were Tumbler and F. Hick - Death Certificate Registration 20/1956, Roebourne district - Banjo (indigent) male age 75 born (c1881) at Mulga Downs, child of Peter (occupation unknown) and Polly spouse not recorded, children Ada (age unknown), date of death 08/12/1956, deceased at Mulga Downs Station, buried at Native Portion Mulga Downs cemetery, last resident at Mulga Downs Station, Wittenoom prior to death. This could possibly be a record of Banjo shown by Palmer to be a partner of ivy Tucker @ Naijong : p. 26 Ivy Tucker shown as mother of Harold (father Harold Mayor, Euro, partner of Daphne Jones); Selina (father Gayuna), Blanche Tucker (father Gayuna), Gertie (father Gayuna), Eric Cosmo (father Spiro Cosmos, Greek); Selina (father Gayuna, partner of Pat Long), Blanche Tucker (father Gayuna), Gertie (father Gayuna, partner of Ginger Parker), John Douglas MacArthur (father McArthur, Euro); Eustace (partner of Dulcie Tumbler) and Ivy also partner of Spider (Ngarla man, no children), Banjo (no children) and Hickey Bung. - Death Certificate Registration 18/1956, Roebourne district - Sally Dundy (indigent native) female age 80 born (c1876) at Mulga Downs, Wittenoom, child of unknown parents, spouse not recorded, no children recorded, date of death 19/10/1956, deceased at Mulga Downs Station, buried at Native Portion Mulga Downs cemetery, last resident at Mulga Downs station, Wittenoom prior to death. -

Foraging Modes of Carnivorous Plants Aaron M
Israel Journal of Ecology & Evolution, 2020 http://dx.doi.org/10.1163/22244662-20191066 Foraging modes of carnivorous plants Aaron M. Ellison* Harvard Forest, Harvard University, 324 North Main Street, Petersham, Massachusetts, 01366, USA Abstract Carnivorous plants are pure sit-and-wait predators: they remain rooted to a single location and depend on the abundance and movement of their prey to obtain nutrients required for growth and reproduction. Yet carnivorous plants exhibit phenotypically plastic responses to prey availability that parallel those of non-carnivorous plants to changes in light levels or soil-nutrient concentrations. The latter have been considered to be foraging behaviors, but the former have not. Here, I review aspects of foraging theory that can be profitably applied to carnivorous plants considered as sit-and-wait predators. A discussion of different strategies by which carnivorous plants attract, capture, kill, and digest prey, and subsequently acquire nutrients from them suggests that optimal foraging theory can be applied to carnivorous plants as easily as it has been applied to animals. Carnivorous plants can vary their production, placement, and types of traps; switch between capturing nutrients from leaf-derived traps and roots; temporarily activate traps in response to external cues; or cease trap production altogether. Future research on foraging strategies by carnivorous plants will yield new insights into the physiology and ecology of what Darwin called “the most wonderful plants in the world”. At the same time, inclusion of carnivorous plants into models of animal foraging behavior could lead to the development of a more general and taxonomically inclusive foraging theory. -

Status of Insectivorous Plants in Northeast India
Technical Refereed Contribution Status of insectivorous plants in northeast India Praveen Kumar Verma • Shifting Cultivation Division • Rain Forest Research Institute • Sotai Ali • Deovan • Post Box # 136 • Jorhat 785 001 (Assam) • India • [email protected] Jan Schlauer • Zwischenstr. 11 • 60594 Frankfurt/Main • Germany • [email protected] Krishna Kumar Rawat • CSIR-National Botanical Research Institute • Rana Pratap Marg • Lucknow -226 001 (U.P) • India Krishna Giri • Shifting Cultivation Division • Rain Forest Research Institute • Sotai Ali • Deovan • Post Box #136 • Jorhat 785 001 (Assam) • India Keywords: Biogeography, India, diversity, Red List data. Introduction There are approximately 700 identified species of carnivorous plants placed in 15 genera of nine families of dicotyledonous plants (Albert et al. 1992; Ellison & Gotellli 2001; Fleischmann 2012; Rice 2006) (Table 1). In India, a total of five genera of carnivorous plants are reported with 44 species; viz. Utricularia (38 species), Drosera (3), Nepenthes (1), Pinguicula (1), and Aldrovanda (1) (Santapau & Henry 1976; Anonymous 1988; Singh & Sanjappa 2011; Zaman et al. 2011; Kamble et al. 2012). Inter- estingly, northeastern India is the home of all five insectivorous genera, namely Nepenthes (com- monly known as tropical pitcher plant), Drosera (sundew), Utricularia (bladderwort), Aldrovanda (waterwheel plant), and Pinguicula (butterwort) with a total of 21 species. The area also hosts the “ancestral false carnivorous” plant Plumbago zelayanica, often known as murderous plant. Climate Lowland to mid-altitude areas are characterized by subtropical climate (Table 2) with maximum temperatures and maximum precipitation (monsoon) in summer, i.e., May to September (in some places the highest temperatures are reached already in April), and average temperatures usually not dropping below 0°C in winter. -

Phytotaxa 2: 46–48 (2009) Review of Pitcher Plants of the Old World
Phytotaxa 2: 46–48 (2009) ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ Book review PHYTOTAXA Copyright © 2009 • Magnolia Press ISSN 1179-3163 (online edition) Review of Pitcher Plants of the Old World MAARTEN J.M. CHRISTENHUSZ1 & MICHAEL F. FAY2 1 Department of Botany, Natural History Museum, Cromwell Road, London SW7 5BD, UK; email [email protected] 2 Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AB, UK; email [email protected] By Stewart McPherson, Pitcher Plants of the Old World, edited by Alastair Robinson and Andreas Fleischmann. Redfern Natural History Productions, Poole, U.K. 2009. Two volumes, 1399 pp. ISBN 978-0-9558918-2-3 and 978-0-9558918-3-0. Publishers price £34.99 each volume. Carnivorous plants have fascinated humans since early history, and these plants continue to tickle the imagination of current day writers. Stewart McPherson shares his fascination for carnivorous plants and he has published various earlier works on the subject, including the excellent Pitcher Plants of the Americas (McPherson, 2006) and Glistening Carnivores (McPherson, 2008), where, as in the current two volumes, many carnivorous plants are described and beautifully illustrated with photographs taken by the author during his intensive field work in often challenging countries and stunning localities. These two volumes cover the pitcher plants from Madagascar, tropical Asia and Australia: the genera Nepenthes Linnaeus (1753: 955), Nepenthaceae, and the south-western Australian endemic Cephalotus follicularis Labilladière (1806: 6) of the peculiar monotypic family Cephalotaceae. The first chapters introduce carnivorous plants in general and illustrate their fascinating trapping mechanisms. The author then introduces pitcher plants of the Old World and discusses relationships with other organisms coexisting with pitcher plants rather than being consumed by them. -

Brooklyn, Cloudland, Melsonby (Gaarraay)
BUSH BLITZ SPECIES DISCOVERY PROGRAM Brooklyn, Cloudland, Melsonby (Gaarraay) Nature Refuges Eubenangee Swamp, Hann Tableland, Melsonby (Gaarraay) National Parks Upper Bridge Creek Queensland 29 April–27 May · 26–27 July 2010 Australian Biological Resources Study What is Contents Bush Blitz? Bush Blitz is a four-year, What is Bush Blitz? 2 multi-million dollar Abbreviations 2 partnership between the Summary 3 Australian Government, Introduction 4 BHP Billiton and Earthwatch Reserves Overview 6 Australia to document plants Methods 11 and animals in selected properties across Australia’s Results 14 National Reserve System. Discussion 17 Appendix A: Species Lists 31 Fauna 32 This innovative partnership Vertebrates 32 harnesses the expertise of many Invertebrates 50 of Australia’s top scientists from Flora 62 museums, herbaria, universities, Appendix B: Threatened Species 107 and other institutions and Fauna 108 organisations across the country. Flora 111 Appendix C: Exotic and Pest Species 113 Fauna 114 Flora 115 Glossary 119 Abbreviations ANHAT Australian Natural Heritage Assessment Tool EPBC Act Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth) NCA Nature Conservation Act 1992 (Queensland) NRS National Reserve System 2 Bush Blitz survey report Summary A Bush Blitz survey was conducted in the Cape Exotic vertebrate pests were not a focus York Peninsula, Einasleigh Uplands and Wet of this Bush Blitz, however the Cane Toad Tropics bioregions of Queensland during April, (Rhinella marina) was recorded in both Cloudland May and July 2010. Results include 1,186 species Nature Refuge and Hann Tableland National added to those known across the reserves. Of Park. Only one exotic invertebrate species was these, 36 are putative species new to science, recorded, the Spiked Awlsnail (Allopeas clavulinus) including 24 species of true bug, 9 species of in Cloudland Nature Refuge. -

Driving Holidays in the Northern Territory the Northern Territory Is the Ultimate Drive Holiday Destination
Driving holidays in the Northern Territory The Northern Territory is the ultimate drive holiday destination A driving holiday is one of the best ways to see the Northern Territory. Whether you are a keen adventurer longing for open road or you just want to take your time and tick off some of those bucket list items – the NT has something for everyone. Top things to include on a drive holiday to the NT Discover rich Aboriginal cultural experiences Try tantalizing local produce Contents and bush tucker infused cuisine Swim in outback waterholes and explore incredible waterfalls Short Drives (2 - 5 days) Check out one of the many quirky NT events A Waterfall hopping around Litchfield National Park 6 Follow one of the unique B Kakadu National Park Explorer 8 art trails in the NT C Visit Katherine and Nitmiluk National Park 10 Immerse in the extensive military D Alice Springs Explorer 12 history of the NT E Uluru and Kings Canyon Highlights 14 F Uluru and Kings Canyon – Red Centre Way 16 Long Drives (6+ days) G Victoria River region – Savannah Way 20 H Kakadu and Katherine – Nature’s Way 22 I Katherine and Arnhem – Arnhem Way 24 J Alice Springs, Tennant Creek and Katherine regions – Binns Track 26 K Alice Springs to Darwin – Explorers Way 28 Parks and reserves facilities and activities 32 Festivals and Events 2020 36 2 Sealed road Garig Gunak Barlu Unsealed road National Park 4WD road (Permit required) Tiwi Islands ARAFURA SEA Melville Island Bathurst VAN DIEMEN Cobourg Island Peninsula GULF Maningrida BEAGLE GULF Djukbinj National Park Milingimbi -

Bushfire Brigade Annual General Meeting
BUSHFIRE BRIGADE ANNUAL GENERAL MEETING AGENDA FOR THE SHIRE OF MINGENEW BUSHFIRE BRIGADES’ ANNUAL GENERAL MEETING TO BE HELD AT THE SHIRE CHAMBERS ON 25 MARCH 2019 COMMENCING AT 6PM. 1.0 DECLARATION OF OPENING 2.0 RECORD OF ATTENDANCE / APOLOGIES ATTENDEES To be confirmed APOLOGIES Vicki Booth – A/Area Officer – Fire Services Midwest (DFES) 3.0 CONFIRMATION OF PREVIOUS MEETING MINUTES 3.1 BUSHFIRE BRIGADES’ MEETING HELD 02 OCTOBER 2018 BRIGADES’ DECISION – ITEM 3.1 Moved: Seconded: That the minutes of the Bushfire Brigades’ Annual General Meeting of the Shire of Mingenew held 02 October 2018 be confirmed as a true and accurate record of proceedings. VOTING DETAILS: 4.0 OFFICERS REPORTS 4.1 Chief Bush Fire Control Officer Report- Murray Thomas • Overview of the 2018/19 Fire Season • Gazetted change in Shires Restricted Burning Times- now changed from the 17th September to the 1st October. All other timeframes remain the same (Prohibited- 1 Nov- 31 Jan, Restricted 1 October-15 March, open season 16 March- 30 September). This means that the CBFCO can now shorten or lengthen that new restricted date by 14 days depending on seasonal conditions (so restricted timeframe can potentially be pushed out to 17 September-31 October or shortened to 14 October-31 October). 4.2 Captains Reports- All Captains to remark on level of training of its volunteers and any identified gaps or training requirements. MINGENEW BUSHFIRE ADVISORY COMMITTEE MEETING AGENDA – 26 September 2017 4.2.1 Yandanooka 4.2.2 Lockier 4.2.3 Guranu 4.2.4 Mingenew North 4.2.5 Mingenew Town 4.3 Shire CEO Report • 2017/18 Operating Grant has been fully expended and acquitted. -

The First Record of the Boreal Bog Species Drosera Rotundifolia (Droseraceae) from the Philippines, and a Key to the Philippine Sundews
Blumea 61, 2016: 24–28 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE http://dx.doi.org/10.3767/000651916X691330 The first record of the boreal bog species Drosera rotundifolia (Droseraceae) from the Philippines, and a key to the Philippine sundews F.P. Coritico1, A. Fleischmann2 Key words Abstract Drosera rotundifolia, a species of the temperate Northern Hemisphere with a disjunct occurrence in high montane West Papua, has been discovered in a highland peat bog on Mt Limbawon, Pantaron Range, Bukidnon carnivorous plants on the island of Mindanao, Philippines, which mediates to the only other known tropical, Southern Hemisphere Drosera location in New Guinea and the closest known northern populations in southern Japan and south-eastern China. Droseraceae A dichotomous key to the seven Drosera species of the Philippines is given, and distribution maps are provided. Malesia Mindanao Published on 15 March 2016 Northern Hemisphere - Tropics disjunction Philippines INTRODUCTION Drosera rotundifolia L. (the generic type) is a temperate, winter dormant species that is widespread in the Northern The Philippines are rich in carnivorous plants, with about 47 Hemisphere, from Pacific North America across large parts of species known from the islands, most of which belong to the northern America and Europe to Siberia and the Kamchatka pitcher plant genus Nepenthes L. This genus has more than 30 Peninsula, South Korea and Japan. It is the Drosera spe- species in the Philippines, all except Nepenthes mirabilis (Lour.) cies covering the largest range, spanning the entire Northern Druce endemic to the country. Most species occur on Mindanao Hemisphere from 180° Western Longitude to about 180° East, and Palawan, while several are confined to a single highland however, not forming a continuous circumboreal range (Diels or even mountain peak (Robinson et al. -

Table 5.8 – Descriptions of Priority Flora Recorded in the Project Area Species, Family and Rank Descriptio
Oakajee Port and Rail OPR Rail Proposal – Vegetation and Flora Assessment Table 5.8 – Descriptions of Priority Flora Recorded in the Project Area No of No of Plants Species, Family and Locations Description Typical Habitat Recorded by Distribution Photographs Rank Recorded by ecologia ecologia A dense, rounded shrub growing from 0.5 m to 2 m in height. Its phyllodes are erect and the yellow, globular flowers are produced from June to August. Acacia lineolata Mullewa, east of subsp. multilineata (Photograph on right by S.J. Patrick. Image used Mingenew, Arrino with the permission of the Western Australian Sandplains. 1 1 (FABACEAE) and the locality of Herbarium, Department of Environment and Yuna. Priority 1 Conservation (http://florabase.dec.wa.gov.au/help/copyright). Accessed on Thursday, 3 December 2009) and growth habit again (right) (Photography: ecologia). Chamelaucium sp. 73 km south of Yalgoo (Y. Chadwick A bushy low shrub to 1.5 m high. This species Yalgoo, near Blue 1816) produces white/pink/purple flowers during Granite outcrops. 2 3 Hills, along Morawa‐ (MYRTACEAE) August and September. Yalgoo Road, and Wurarga. Priority 1 An upright, leafless, semi‐succulent herb that grows to between 0.4 and 1 m, although it has been recorded as growing to 2 m. The stems are Robinson Ranges, Euphorbia light green, and have a bluish‐grey waxy light Sandstone and Mount Augustus sarcostemmoides covering. When broken a white sap is exuded quartzite hills but Station. Also East from the stems. The rarely present leaves are has been located 67 254 Chewing Ranges, (EUPHORBIACEAE) narrow, lanceolate, opposite and are held on flat plains at Mount Giles and Priority 1 horizontally. -
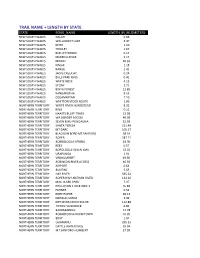
Trail Name + Length by State
TRAIL NAME + LENGTH BY STATE STATE ROAD_NAME LENGTH_IN_KILOMETERS NEW SOUTH WALES GALAH 0.66 NEW SOUTH WALES WALLAGOOT LAKE 3.47 NEW SOUTH WALES KEITH 1.20 NEW SOUTH WALES TROLLEY 1.67 NEW SOUTH WALES RED LETTERBOX 0.17 NEW SOUTH WALES MERRICA RIVER 2.15 NEW SOUTH WALES MIDDLE 40.63 NEW SOUTH WALES NAGHI 1.18 NEW SOUTH WALES RANGE 2.42 NEW SOUTH WALES JACKS CREEK AC 0.24 NEW SOUTH WALES BILLS PARK RING 0.41 NEW SOUTH WALES WHITE ROCK 4.13 NEW SOUTH WALES STONY 2.71 NEW SOUTH WALES BINYA FOREST 12.85 NEW SOUTH WALES KANGARUTHA 8.55 NEW SOUTH WALES OOLAMBEYAN 7.10 NEW SOUTH WALES WHITTON STOCK ROUTE 1.86 NORTHERN TERRITORY WAITE RIVER HOMESTEAD 8.32 NORTHERN TERRITORY KING 0.53 NORTHERN TERRITORY HAASTS BLUFF TRACK 13.98 NORTHERN TERRITORY WA BORDER ACCESS 40.39 NORTHERN TERRITORY SEVEN EMU‐PUNGALINA 52.59 NORTHERN TERRITORY SANTA TERESA 251.49 NORTHERN TERRITORY MT DARE 105.37 NORTHERN TERRITORY BLACKGIN BORE‐MT SANFORD 38.54 NORTHERN TERRITORY ROPER 287.71 NORTHERN TERRITORY BORROLOOLA‐SPRING 63.90 NORTHERN TERRITORY REES 0.57 NORTHERN TERRITORY BOROLOOLA‐SEVEN EMU 32.02 NORTHERN TERRITORY URAPUNGA 1.91 NORTHERN TERRITORY VRDHUMBERT 49.95 NORTHERN TERRITORY ROBINSON RIVER ACCESS 46.92 NORTHERN TERRITORY AIRPORT 0.64 NORTHERN TERRITORY BUNTINE 5.63 NORTHERN TERRITORY HAY RIVER 335.62 NORTHERN TERRITORY ROPER HWY‐NATHAN RIVER 134.20 NORTHERN TERRITORY MAC CLARK PARK 7.97 NORTHERN TERRITORY PHILLIPSON STOCK ROUTE 55.84 NORTHERN TERRITORY FURNER 0.54 NORTHERN TERRITORY PORT ROPER 40.13 NORTHERN TERRITORY NDHALA GORGE 3.49 NORTHERN TERRITORY -

Fish Fauna of the Fitzroy River in the Kimberley Region of Western Australia - Including the Bunuba, Gooniyandi, Ngarinyin, Nyikina and Walmajarri Aboriginal Names
DOI: 10.18195/issn.0312-3162.22(2).2004.147-161 Records of the Westelll Allstralllll1 A//uselllll 22 ]47-]6] (2004). Fish fauna of the Fitzroy River in the Kimberley region of Western Australia - including the Bunuba, Gooniyandi, Ngarinyin, Nyikina and Walmajarri Aboriginal names J J 2 3 David L. Morgan , Mark G. Allen , Patsy Bedford and Mark Horstman 1 Centre for Fish & Fisheries Research, School of Biological Sciences and Biotechnology, Murdoch University, Murdoch, Western Australia 6]50 KImberley Language Resource Centre, PO Box 86, Fitzroy Crossing, Western Australia 6765 'Kimberley Land Council, PO Box 2145, Broome Western Australia 6725 Abstract - This project surveyed the fish fauna of the Fitzroy River, one of Australia's largest river systems that remains unregulated, 'located in the Kimberley region of Western Australia. A total of 37 fish species were recorded in the 70 sites sampled. Twenty-three of these species are freshwater fishes (i.e. they complete their life-cycle in freshwater), the remainder being of estuarine or marine origin that may spend part of their life-cycle in freshwater. The number of freshwater species in the Fitzroy River is high by Australian standards. Three of the freshwater fish species recorded ar'e currently undescribed, and two have no formal common or scientific names, but do have Aboriginal names. Where possible, the English (common), scientific and Aboriginal names for the different speCIes of the river are given. This includes the Aboriginal names of the fish for the following five languages (Bunuba, Gooniyandi, Ngarinyin, Nyikina and Walmajarri) of the Fitzroy River Valley. The fish fauna of the river was shown to be significantly different between each of the lower, middle and upper reaches of the main channeL Furthermore, the smaller tributaries and the upper gorge country sites were significantly different to those in the main channel, while the major billabongs of the river had fish assemblages significantly different to all sites with the exception of the middle reaches of the river. -

631 ROBERT and CHARLES Robert and I Were Sitting Beside His
ROBERT AND CHARLES Robert and I were sitting beside his pool at his home above Aspen looking across at the mountains. We had just had lunch under the shade of the native scrub oaks. It was a beautiful sunny autumn day and the leaves had started to change colour. We were feeling pretty good as we had just finished building the colossal edition of my sculpture Creation out of 10-foot long Oregon planks. Everything had gone well and we both agreed that the sculpture looked great! Robert asked me about the trip that I had just done with Damon and his family to Mt Agnes. I told him the story, including our chance meeting with Grahame Walsh, and the mystery of the missing paintings. I raved on about Mount Agnes, about how I felt that I had some kind of affinity with the place and ended by saying that if I had one wish in my life, it would be to sleep naked on top of Mountt Agnes under a full moon. Well, sometimes you can get carried away! Robert looked across at me and said, "Why don’t we?" I was speechless and nearly fell off my chair. He went on to explain that anything that I spoke so passionately about, he wanted to experience for himself! He also would like to spend some time with his 30-year-old son, Charles, and what better place to do it than on a trip into the Outback. He had never been to Australia and he would like to see some Wandjina paintings and Kimberley before it was ruined by tourism, as it soon surely would be.