R-410A Refrigerant Date Prepared: 05-2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1,1,1,2-Tetrafluoroethane
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Concise International Chemical Assessment Document 11 1,1,1,2-Tetrafluoroethane First draft prepared by Mrs P. Barker and Mr R. Cary, Health and Safety Executive, Liverpool, United Kingdom, and Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United Kingdom Please not that the layout and pagination of this pdf file are not identical to the printed CICAD Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 1998 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organisation (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. The Inter-Organization -

SAFETY DATA SHEET Difluoromethane (R32) SECTION 1
SAFETY DATA SHEET Difluoromethane (R32) Issue Date: 16.01.2013 Version: 1.1 SDS No.: 000010021734 Last revised date: 26.11.2018 1/14 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Product name: Difluoromethane (R32) Other Name: HFC-32 Additional identification Chemical name: Difluoromethane Chemical formula: CH2F2 INDEX No. - CAS-No. 75-10-5 EC No. 200-839-4 REACH Registration No. 01-2119471312-47 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Industrial and professional. Perform risk assessment prior to use. Refrigerant. Use as an Intermediate (transported, on-site isolated). Use for electronic component manufacture. Using gas alone or in mixtures for the calibration of analysis equipment. Formulation of mixtures with gas in pressure receptacles. Uses advised against Consumer use. 1.3 Details of the supplier of the safety data sheet Supplier Linde Gas GmbH Telephone: +43 50 4273 Carl-von-Linde-Platz 1 A-4651 Stadl-Paura E-mail: [email protected] 1.4 Emergency telephone number: Emergency number Linde: + 43 50 4273 (during business hours), Poisoning Information Center: +43 1 406 43 43 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 as amended. Physical Hazards Flammable gas Category 1 H220: Extremely flammable gas. Gases under pressure Liquefied gas H280: Contains gas under pressure; may explode if heated. SDS_AT - 000010021734 SAFETY DATA SHEET Difluoromethane (R32) Issue Date: 16.01.2013 Version: 1.1 SDS No.: 000010021734 Last revised date: 26.11.2018 2/14 2.2 Label Elements Signal Words: Danger Hazard Statement(s): H220: Extremely flammable gas. -

Purification Process of Pentafluoroethane (HFC-125) Verfahren Zur Reinigung Von Pentafluorethan (HFC-125) Procédé De Purification Du Pentafluoroéthane (HFC-125)
Europäisches Patentamt *EP001153907B1* (19) European Patent Office Office européen des brevets (11) EP 1 153 907 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.7: C07C 17/386 of the grant of the patent: 06.10.2004 Bulletin 2004/41 (21) Application number: 01109907.4 (22) Date of filing: 24.04.2001 (54) Purification process of pentafluoroethane (HFC-125) Verfahren zur Reinigung von Pentafluorethan (HFC-125) Procédé de purification du pentafluoroéthane (HFC-125) (84) Designated Contracting States: • Basile, Giampiero DE ES FR GB IT NL 15100 Alessandria (IT) (30) Priority: 09.05.2000 IT MI001006 (74) Representative: Jacques, Philippe et al Solvay S.A. (43) Date of publication of application: Département de la Propriété Industrielle, 14.11.2001 Bulletin 2001/46 Rue de Ransbeek, 310 1120 Bruxelles (BE) (73) Proprietor: Solvay Solexis S.p.A. 20121 Milano (IT) (56) References cited: EP-A- 0 626 362 EP-A- 0 985 650 (72) Inventors: • Azzali, Daniele 20021 Bollate (MI) (IT) Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall be filed in a written reasoned statement. It shall not be deemed to have been filed until the opposition fee has been paid. (Art. 99(1) European Patent Convention). EP 1 153 907 B1 Printed by Jouve, 75001 PARIS (FR) EP 1 153 907 B1 Description [0001] The present invention relates to a purification process of pentafluoroethane (HFC-125) containing as impurity chloropentafluoroethane (CFC-115). -
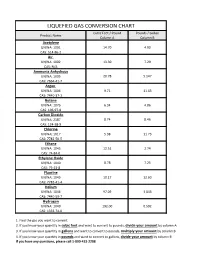
Liquefied Gas Conversion Chart
LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Acetylene UN/NA: 1001 14.70 4.90 CAS: 514-86-2 Air UN/NA: 1002 13.30 7.29 CAS: N/A Ammonia Anhydrous UN/NA: 1005 20.78 5.147 CAS: 7664-41-7 Argon UN/NA: 1006 9.71 11.63 CAS: 7440-37-1 Butane UN/NA: 1075 6.34 4.86 CAS: 106-97-8 Carbon Dioxide UN/NA: 2187 8.74 8.46 CAS: 124-38-9 Chlorine UN/NA: 1017 5.38 11.73 CAS: 7782-50-5 Ethane UN/NA: 1045 12.51 2.74 CAS: 74-84-0 Ethylene Oxide UN/NA: 1040 8.78 7.25 CAS: 75-21-8 Fluorine UN/NA: 1045 10.17 12.60 CAS: 7782-41-4 Helium UN/NA: 1046 97.09 1.043 CAS: 7440-59-7 Hydrogen UN/NA: 1049 192.00 0.592 CAS: 1333-74-0 1. Find the gas you want to convert. 2. If you know your quantity in cubic feet and want to convert to pounds, divide your amount by column A 3. If you know your quantity in gallons and want to convert to pounds, multiply your amount by column B 4. If you know your quantity in pounds and want to convert to gallons, divide your amount by column B If you have any questions, please call 1-800-433-2288 LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Hydrogen Chloride UN/NA: 1050 10.60 8.35 CAS: 7647-01-0 Krypton UN/NA: 1056 4.60 20.15 CAS: 7439-90-9 Methane UN/NA: 1971 23.61 3.55 CAS: 74-82-8 Methyl Bromide UN/NA: 1062 4.03 5.37 CAS: 74-83-9 Neon UN/NA: 1065 19.18 10.07 CAS: 7440-01-9 Mapp Gas UN/NA: 1060 9.20 4.80 CAS: N/A Nitrogen UN/NA: 1066 13.89 6.75 CAS: 7727-37-9 Nitrous Oxide UN/NA: 1070 8.73 6.45 CAS: 10024-97-2 Oxygen UN/NA: 1072 12.05 9.52 CAS: 7782-44-7 Propane UN/NA: 1075 8.45 4.22 CAS: 74-98-6 Sulfur Dioxide UN/NA: 1079 5.94 12.0 CAS: 7446-09-5 Xenon UN/NA: 2036 2.93 25.51 CAS: 7440-63-3 1. -

RS44B (R453A) Safety Data Sheet
RS44B (R453A) Safety Data Sheet 06/01/2015 SECTION 1: IDENTIFICATION Product identifier Product Form: Mixture Product Name: In USA as RS44B (R453A) Alternate Names: Blended FormulaIntended Use of the Product Refrigerant Name, Address, and Telephone of the Responsible Party Company ComStar International Inc. 20-45 128th Street, College Point, NY 11356 Emergency Telephone Number Emergency number :(800) 328-0142, (718) 445-7900 SECTION 2: HAZARDS IDENTIFICATION Classification of the Substance or Mixture Classification (GHS-US) Simple Asphyxiant Liquefied gas H280 Label Elements GHS-US Labeling Hazard Pictograms (GHS-US) : Signal Word (GHS-US) : Warning Hazard Statements (GHS-US) : H280 - Contains gas under pressure; may explode if heated May displace oxygen and cause rapid suffocation Precautionary Statements (GHS-US) : P410+P403 - Protect from sunlight. Store in a well-ventilated place Other Hazardsx Other Hazards Not Contributing to the Classification: Exposure may aggravate those with pre-existing eye, skin, or respiratory conditions. Liquid contact with eyes or skin may cause frostbite. Unknown Acute Toxicity (GHS-US) Not available SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTS Substances-Name Product identifier % (w/W) Classification (GHS-US) 1,1,1,2,3,3,3 Heptafluoropropane (HFC 227) (CAS No) 431-89-0 5 Simple Asphyxiant Liquefied gas, H280 Pentafluoroethane (HFC125) (CAS No) 354-33-6 20 Simple Asphyxiant Liquefied gas, H280 1,1,1,2-Tetrafluoroethane (HFC-134a) (CAS No) 811-97-2 53.8 Simple Asphyxiant Liquefied gas, H280 Difluoromethane (HFC-32) (CAS No) 75-10-5 20 Simple Asphyxiant Flam. Gas 1, H220 Liquefied gas, H280 Butane (HC-R600) (CAS No) 106-97-8 0.6 Simple Asphyxiant Flam. -

Gas Conversion Factor for 300 Series
300GasTable Rec # Gas Symbol GCF Density (g/L) Density (g/L) 25° C / 1 atm 0° C / 1 atm 1 Acetic Acid C2H4F2 0.4155 2.7 2.947 2 Acetic Anhydride C4H6O3 0.258 4.173 4.555 3 Acetone C3H6O 0.3556 2.374 2.591 4 Acetonitryl C2H3N 0.5178 1.678 1.832 5 Acetylene C2H2 0.6255 1.064 1.162 6 Air Air 1.0015 1.185 1.293 7 Allene C3H4 0.4514 1.638 1.787 8 Ammonia NH3 0.7807 0.696 0.76 9 Argon Ar 1.4047 1.633 1.782 10 Arsine AsH3 0.7592 3.186 3.478 11 Benzene C6H6 0.3057 3.193 3.485 12 Boron Trichloride BCl3 0.4421 4.789 5.228 13 Boron Triflouride BF3 0.5431 2.772 3.025 14 Bromine Br2 0.8007 6.532 7.13 15 Bromochlorodifluoromethane CBrClF2 0.3684 6.759 7.378 16 Bromodifluoromethane CHBrF2 0.4644 5.351 5.841 17 Bromotrifluormethane CBrF3 0.3943 6.087 6.644 18 Butane C4H10 0.2622 2.376 2.593 19 Butanol C4H10O 0.2406 3.03 3.307 20 Butene C4H8 0.3056 2.293 2.503 21 Carbon Dioxide CO2 0.7526 1.799 1.964 22 Carbon Disulfide CS2 0.616 3.112 3.397 23 Carbon Monoxide CO 1.0012 1.145 1.25 24 Carbon Tetrachloride CCl4 0.3333 6.287 6.863 25 Carbonyl Sulfide COS 0.668 2.456 2.68 26 Chlorine Cl2 0.8451 2.898 3.163 27 Chlorine Trifluoride ClF3 0.4496 3.779 4.125 28 Chlorobenzene C6H5Cl 0.2614 4.601 5.022 29 Chlorodifluoroethane C2H3ClF2 0.3216 4.108 4.484 30 Chloroform CHCl3 0.4192 4.879 5.326 31 Chloropentafluoroethane C2ClF5 0.2437 6.314 6.892 32 Chloropropane C3H7Cl 0.308 3.21 3.504 33 Cisbutene C4H8 0.3004 2.293 2.503 34 Cyanogen C2N2 0.4924 2.127 2.322 35 Cyanogen Chloride ClCN 0.6486 2.513 2.743 36 Cyclobutane C4H8 0.3562 2.293 2.503 37 Cyclopropane C3H6 0.4562 -

Cclf3), CFC-114 (C 2Cl2f4), and CFC-115 (C2clf5
Atmos. Chem. Phys., 18, 979–1002, 2018 https://doi.org/10.5194/acp-18-979-2018 © Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Atmospheric histories and emissions of chlorofluorocarbons CFC-13 (CClF3), 6CFC-114 (C2Cl2F4), and CFC-115 (C2ClF5) Martin K. Vollmer1, Dickon Young2, Cathy M. Trudinger3, Jens Mühle4, Stephan Henne1, Matthew Rigby2, Sunyoung Park5, Shanlan Li5, Myriam Guillevic6, Blagoj Mitrevski3, Christina M. Harth4, Benjamin R. Miller7,8, Stefan Reimann1, Bo Yao9, L. Paul Steele3, Simon A. Wyss1, Chris R. Lunder10, Jgor Arduini11,12, Archie McCulloch2, Songhao Wu5, Tae Siek Rhee13, Ray H. J. Wang14, Peter K. Salameh4, Ove Hermansen10, Matthias Hill1, Ray L. Langenfelds3, Diane Ivy15, Simon O’Doherty2, Paul B. Krummel3, Michela Maione11,12, David M. Etheridge3, Lingxi Zhou16, Paul J. Fraser3, Ronald G. Prinn15, Ray F. Weiss4, and Peter G. Simmonds2 1Laboratory for Air Pollution and Environmental Technology, Empa, Swiss Federal Laboratories for Materials Science and Technology, Überlandstrasse 129, 8600 Dübendorf, Switzerland 2Atmospheric Chemistry Research Group, School of Chemistry, University of Bristol, Bristol, UK 3Climate Science Centre, CSIRO Oceans and Atmosphere, Aspendale, Victoria, Australia 4Scripps Institution of Oceanography, University of California at San Diego, La Jolla, California, USA 5Kyungpook Institute of Oceanography, Kyungpook National University, South Korea 6METAS, Federal Institute of Metrology, Lindenweg 50, Bern-Wabern, Switzerland 7Earth System Research -

Safety Data Sheet Pentafluoroethane 1. Identification of the Substance/Compound and Company 2. Hazards Identification
Revised edition no : 4 SAFETY DATA SHEET ACCORDING TO REGULATION 1907/2006 Date : 08.10.2019 PENTAFLUOROETHANE 1. IDENTIFICATION OF THE SUBSTANCE/COMPOUND AND COMPANY 1.1. Product identifier Name: Pentafluoroethane Chemical name IUPAC name: 1,1,1,2,2-pentafluoroethane Refrigerant-125 Freon-125 HP, refrigerator gas R 125 GHFU-125 Synonyms: R 125, HFC 125 Chemical formula: C2F5H Molecular weight: 120,0214 EC number 206-557-8 REACH Pre-Registration № Reference number 05-2114096899-20-0000 C&L bulk notification Reference number 02-2119708817-31-0000 CAS number 354-33-6 Structural formula: 1.2. Use of substance/ Applied as refrigerant both separate and in mixture composition; in sprinklers compound as fire-extinguishing means Identified uses Manufacture of substance Formulation/Blending Packaging/repackaging Manufacture of charged RAC/MAC systems and other refrigeration machines Manufacture of fire extinguishers Recovery operations = Recycling / Reclamation / Destruction (waste) MAC & RAC Mobile Air Conditoning and Stationary Refrigeration & Air Conditioning systems Recovery (F-gas / ODS) / Servicing Most common technical function of substance (what it does): Heat transfer agent Laboratory chemicals Other: Fire extinguishing agent Uses advised against For industrial or professional use only 1.3. Details of the supplier of the safety data sheet Manufacturer Joint Stock Company «HaloPolymer Perm» 614042, Russia, Perm, ul. Lasvinskaya 98 Phone № +7(342) 250-61-50 www.halopolymer.ru Only REACH representative in JSC «HaloPolymer Perm» (Submitting legal entity URALCHEM Assist EU: GmbH) Johannssenstrasse 10 30159, Hannover, Germany Tel: +49 511 45 99 444 1.4 Emergency telephone: +7-342-282-85-45 (24 hours) Great Britain +44 (0) 203 394 9870 (24/7) USA 1-877 271 7077 2. -
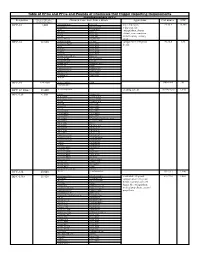
Table of Hfcs and Pfcs and Pounds of Chemicals That Trigger
Table of HFCs and PFCs and Pounds of Chemicals that Trigger Reporting Requirements Hydrofluorocarbons (HFCs) Designation Pounds of Substance = Chemical Name, Trade Names, Blends Applications CAS number GWP 10,000 metric tons Ce HFC-23 1,480 trifluoromethane Suva 95 low temperature 75-46-7 14,800 fluoroform Genetron 503 refrigerant, fire R-23 Genetron 23 extinguishant, plasma Freon 23 Klea 23 FE-13 Klea 508 etchant, semi-conductor FE-36 Klea 5R3 manufacturing cleaning Forane FX 220 NARM 503 agent HFC-32 32,660 difluoromethane R-407C component in refrigerant 75-10-5 675 methylene fluoride Klea 407A blends R-32 Klea 407B R-410A Klea 407C R-407C Klea 407D Freon 32 Klea 410A Forane 410A (AZ-20) Klea 32 Forane FX 40 Genetron 407C Forane FX 220 AZ-20 Forane 407C EcoloAce 407c Forane 32 HX4 Asahiklin SA-39 Solkane 407C Asahiklin SA-45 Solkane 410 Meforex 98 Suva 9000 Meforex 32 Suva 9100 R-410A HFC-41 227,280 methyl fluoride R-41 593-53-3 97 fluoromethane HFC-43-10mee 13,440 decafluoropentane cleaning solvent 138495-42-8 1,640 HFC-125 6,300 ethane Forane 507 pentafluoro Forane FX10 pentafluoroethane Forane FX40 R-125 Forane FX70 FC-125 Suva 125 Freon 125 Suva 9000 FE-25 Suva 9100 Arcton 402A Suva HP62 Arcton 402B Suva HP80 Arcton 408A Suva HP81 Klea 404A Cooltop R-134a Replacement Klea 407A EcoloAce 404a Klea 407B Di 44 Klea 407C Meforex 125 Klea 407D Meforex 55 Klea 410A Meforex 57 Klea 507A Meforex 98 Asahiklin SA-28 ISCEON 29 Asahiklin SA-39 ISCEON 404A Asahiklin SA-45 ISCEON 507 AZ-20 ISCEON 59 AZ-50 ISCEON 79 Genetron 404A ISCEON 89 Genetron -

R32 Common Questions
Frequently Asked Questions 1. How will the new F-gas regulation affect the choice of refrigerant for heat pump air conditioners? Heat pump air conditioning systems are impacted by the new F-gas regulation, the gradual phase down of HFC’s quantities on the market will push the industry to use lower GWP refrigerants. Since 2017 the price of HFC’s has started to rise significantly, especially for those gases with high Global Warming Potential (GWP). Note: In the new F-gas regulation, the use of HFC’s with GWP greater than 750 will be prohibited from 2025 for mono split-system room air conditioners containing less than 3 kg of refrigerant. 2. What is Toshiba’s refrigerant of choice as a replacement for R-410A heat pump air conditioners? R-410A alternatives must provide an acceptable compromise in terms of GWP, human safety, energy efficiency, and system cost. After several years of research and evaluation, Toshiba along with other air conditioner manufacturers are switching from R-410A to R-32 (Difluoromethane HFC32) refrigerant which has a lower Global Warming Potential (GWP), is better for the environment and delivers greater energy efficiency. However this gas is mildly flammable (A2L safety class) and does require some redesign change. These changes will be seen in our new range of R32 residential and light commercial heat pump air conditioning systems. The benefits of HFC R-32 are:- - Zero Ozone Depletion - 1/3 GWP of HFC 410A (GWP675 v GWP2088) - Superior energy efficiency - High refrigeration capacity and thermal conductivity - Low -

Compositions of Difluoromethane, Pentafluoroethane, 1,1,1,2-Tetrafluoroethane and Hydrocarbons
(19) & (11) EP 1 163 313 B2 (12) NEW EUROPEAN PATENT SPECIFICATION After opposition procedure (45) Date of publication and mention (51) Int Cl.: of the opposition decision: C09K 5/04 (2006.01) 04.11.2009 Bulletin 2009/45 (86) International application number: (45) Mention of the grant of the patent: PCT/US2000/007546 06.10.2004 Bulletin 2004/41 (87) International publication number: (21) Application number: 00918231.2 WO 2000/056834 (28.09.2000 Gazette 2000/39) (22) Date of filing: 22.03.2000 (54) COMPOSITIONS OF DIFLUOROMETHANE, PENTAFLUOROETHANE, 1,1,1,2- TETRAFLUOROETHANE AND HYDROCARBONS ZUSAMMENSETZUNGEN AUS DIFLUORMETHAN, PENTAFLUORETHAN, 1,1,1,2- TETRAFLUORETHAN UND KOHLENWASSERSTOFFEN COMPOSITIONS DE DIFLUOROMETHANE, PENTAFLUOROETHANE, 1,1,1,2- TETRAFLUOROETHANE ET D’HYDROCARBURES (84) Designated Contracting States: • YOKOZEKI, Akimichi DE ES FR GB IT NL Wilmington, DE 19807 (US) (30) Priority: 22.03.1999 US 125510 P (74) Representative: Matthews, Derek Peter 21.03.2000 US 528964 Frank B. Dehn & Co. St Bride’s House (43) Date of publication of application: 10 Salisbury Square 19.12.2001 Bulletin 2001/51 London EC4Y 8JD (GB) (73) Proprietor: E.I. DU PONT DE NEMOURS AND COMPANY (56) References cited: Wilmington, DE 19898 (US) EP-A- 0 659 862 EP-A2- 0 563 806 EP-A2- 0 700 988 WO-A-96/03473 (72) Inventors: WO-A-97/15637 WO-A1-94/18282 • BIVENS, Donald, Bernard WO-A1-99/03947 US-A- 5 393 442 Kennett Square, PA 19348 (US) US-A- 5 674 825 • MINOR, Barbara, Haviland Elkton, MD 21921 (US) EP 1 163 313 B2 Printed by Jouve, 75001 PARIS (FR) EP 1 163 313 B2 Description FIELD OF THE INVENTION 5 [0001] The present invention relates to azeotrope-like compositions consisting essentially of difluoromethane, pen- tafluoroethane, 1, 1, 1,2-tetrafluoroethane and a hydrocarbon selected from the group consisting of: n-butane; isobutane; n-butane and 2-methylbutane; n-butane and n-pentane; isobutane and 2-methylbutane; and isobutane and n-pentane. -

Freon™ 407C and 407A Properties, Uses, Storage, and Handling
Freon™ 407C and 407A Refrigerants (R-407C and R-407A) Properties, Uses, Storage, and Handling Freon™ Refrigerants Table of Contents Introduction ...........................................................................4 Air Monitors and Leak Detection ...................................... 16 Freon™ 407C and Freon™ 407A Refrigerant Types of Detectors .................................................................................16 Descriptions ................................................................................................... 4 Nonselective Detectors .................................................................16 Uses ......................................................................................4 Halogen-Selective Detectors ....................................................16 Physical Properties ...............................................................4 Compound-Specific Detectors .................................................16 Fluorescent Additives .....................................................................16 Chemical/Thermal Stability .............................................. 10 Stability with Metals .............................................................................10 Storage and Handling ........................................................ 17 Thermal Decomposition......................................................................11 Shipping Containers in the United States ..............................17 Compatibility Concerns If HCFC-22 and Freon™ Bulk Storage