Breeding and Hibernation of Captive Meadow Jumping Mice (Zapus Hudsonius) 2 3 4 Ethan A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Natural History
NATURAL HISTORY By Kate Warren and Ian MacQuarrie nimals have a variety of gaits, with woods. They are very similar in size and very sensibly, in hibernation. A the pattern employed depending colour: brown backs, yellow sides shad- Aside from the tail tips, what are the upon habitat and circumstance. More- ing to white underneath. The easiest way main differences between the two spe- over, since much knowledge of the life to tell them from the runners is by their cies? Evolution theory suggests that and times of a species comes from inter- tails, which are longer than their bodies. there must be some dissimilarities, or preting tracks, some attention to the It is safe to assume that any mouse with else one would compete strongly against, dancing feet that make them is useful such a long tail is a jumping mouse. and perhaps eliminate, the other. There and often necessary. Basic biology, then, And how do you tell the jumping mice are, of course, differences in preferred is not just counting teeth; it is looking at apart? Again, the tail distinguishes them: habitat between woodland and meadow toes, and how these are picked up and the woodland jumping mouse has a white- jumpers, but they also differ in social put down. tipped tail, the meadow jumping mouse customs, behaviour. Both species are For instance, there are six species of does not. Such minor differences may b e somewhat nomadic, but the grassland small rodents on Prince Edward Island lost on your cat, but they are mighty jumper is usually found alone, while the that can generally be called "mice." handy for naturalists. -

Metro Response to Public Comments Proposed License for Grimm's Fuel
Metro response to public comments Proposed license for Grimm’s Fuel Company Metro response to public comments received during the comment period in October through November 2018 regarding proposed license conditions for Grimm’s Fuel Company. Prepared by Hila Ritter January 4, 2019 BACKGROUND Grimm’s Fuel Company (Grimm’s), is a locally-owned and operated Metro-licensed yard debris composting facility that primarily accepts yard debris for composting. In general, Metro’s licensing requirements for a composting facility set conditions for receiving, managing, and transferring yard debris and other waste at the facility. The Oregon Department of Environmental Quality (DEQ) also has distinct yet complementary requirements for composting facilities and monitors environmental impacts of facilities to protect air, soil, and water quality. Metro and DEQ work closely together to provide regulatory oversight of solid waste facilities such as Grimm’s. On October 22, 2018, Metro opened a formal public comment period for several new conditions of the draft license for Grimm’s. The public comment period closed on November 30, 2018. Metro also hosted a community conversation during that time to further solicit input and discuss next steps. During the formal comment period, Metro received 119 written comments from individuals and businesses (attached). Metro received an additional six written comments via email after the comment period closed at 5 p.m. on November 30, which did not become an official part of the record. After the comment period closed, members of the Tualatin community requested that Metro extend the term of Grimm’s current license for an additional two months to allow the public an opportunity to review the new proposed license in its entirety before it is issued. -

Mandible Variation in the Dwarf Fat-Tailed Jerboa, Pygeretmus Pumilio (Rodentia: Dipodidae)
Canadian Journal of Zoology Phenotypic plasticity under desert environment constraints: mandible variation in the dwarf fat-tailed jerboa, Pygeretmus pumilio (Rodentia: Dipodidae) Journal: Canadian Journal of Zoology Manuscript ID cjz-2019-0029.R1 Manuscript Type: Article Date Submitted by the 17-Apr-2019 Author: Complete List of Authors: Krystufek, Boris; Slovenian Museum of Natural History Janzekovic, Franc; Faculty of Natural Sciences and Mathematics, University of Maribor Shenbrot, DraftGeorgy; Ben-Gurion University of the Negev, Mitrani Department of Desert Ecology Ivajnsic, Danijel; Faculty of Natural Sciences and Mathematics, University of Maribor Klenovsek, Tina; Faculty of Natural Sciences and Mathematics, University of Maribor Is your manuscript invited for consideration in a Special Not applicable (regular submission) Issue?: Bergmann’s rule, desert ecology, ecomorphology, geometric Keyword: morphometrics, dwarf fat-tailed jerboa, Pygeretmus pumilio, resource availability https://mc06.manuscriptcentral.com/cjz-pubs Page 1 of 46 Canadian Journal of Zoology Phenotypic plasticity under desert environment constraints: mandible variation in the dwarf fat-tailed jerboa, Pygeretmus pumilio (Rodentia: Dipodidae) B. Kryštufek, F. Janžekovič, G. Shenbrot, D. Ivajnšič, and T. Klenovšek B. Kryštufek. Slovenian Museum of Natural History, Prešernova 20, 1000 Ljubljana, Slovenia, email: [email protected] F. Janžekovič, D. Ivajnšič, and T. Klenovšek. Faculty of Natural Sciences and Mathematics, University of Maribor, Koroška 160, 2000 Maribor, Slovenia, emails: [email protected]; [email protected]; [email protected] G. Shenbrot. Mitrani Department ofDraft Desert Ecology, Jacob Blaustein Institutes for Desert Research, Ben-Gurion University of the Negev, Midreshet Ben-Gurion, Israel, email: [email protected] Correspondence: T. Klenovšek Address: Faculty of Natural Sciences and Mathematics, Koroška 160, 2000 Maribor, Slovenia. -

Mothers Grimm Kindle
MOTHERS GRIMM PDF, EPUB, EBOOK Danielle Wood | 224 pages | 01 Oct 2016 | Allen & Unwin | 9781741756746 | English | St Leonards, Australia Mothers Grimm PDF Book Showing An aquatic reptilian-like creature that is an exceptional swimmer. They have a temper that they control and release to become effective killers, particularly when a matter involves a family member or loved one. She took Nick to Weston's car and told Nick that he knew Adalind was upstairs with Renard, and the two guys Weston sent around back knew too. When Wu asks how she got over thinking it was real, she tells him that it didn't matter whether it was real, what mattered was losing her fear of it. Dick Award Nominee I found the characters appealing, and the plot intriguing. This wesen is portrayed as the mythological basis for the Three Little Pigs. The tales are very dark, and while the central theme is motherhood, the stories are truly about womanhood, and society's unrealistic and unfair expectations of all of us. Paperback , pages. The series presents them as the mythological basis for The Story of the Three Bears. In a phone call, his parents called him Monroe, seeming to indicate that it is his first name. The first edition contained 86 stories, and by the seventh edition in , had unique fairy tales. Danielle is currently teaching creative writing at the University of Tasmania. The kiss of a musai secretes a psychotropic substance that causes obsessive infatuation. View all 3 comments. He asks Sean Renard, a police captain, to endorse him so he would be elected for the mayor position. -

Genetic Diversity of Bartonella Species in Small Mammals in the Qaidam
www.nature.com/scientificreports OPEN Genetic diversity of Bartonella species in small mammals in the Qaidam Basin, western China Huaxiang Rao1, Shoujiang Li3, Liang Lu4, Rong Wang3, Xiuping Song4, Kai Sun5, Yan Shi3, Dongmei Li4* & Juan Yu2* Investigation of the prevalence and diversity of Bartonella infections in small mammals in the Qaidam Basin, western China, could provide a scientifc basis for the control and prevention of Bartonella infections in humans. Accordingly, in this study, small mammals were captured using snap traps in Wulan County and Ge’ermu City, Qaidam Basin, China. Spleen and brain tissues were collected and cultured to isolate Bartonella strains. The suspected positive colonies were detected with polymerase chain reaction amplifcation and sequencing of gltA, ftsZ, RNA polymerase beta subunit (rpoB) and ribC genes. Among 101 small mammals, 39 were positive for Bartonella, with the infection rate of 38.61%. The infection rate in diferent tissues (spleens and brains) (χ2 = 0.112, P = 0.738) and gender (χ2 = 1.927, P = 0.165) of small mammals did not have statistical diference, but that in diferent habitats had statistical diference (χ2 = 10.361, P = 0.016). Through genetic evolution analysis, 40 Bartonella strains were identifed (two diferent Bartonella species were detected in one small mammal), including B. grahamii (30), B. jaculi (3), B. krasnovii (3) and Candidatus B. gerbillinarum (4), which showed rodent-specifc characteristics. B. grahamii was the dominant epidemic strain (accounted for 75.0%). Furthermore, phylogenetic analysis showed that B. grahamii in the Qaidam Basin, might be close to the strains isolated from Japan and China. -
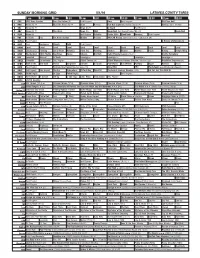
Sunday Morning Grid 5/1/16 Latimes.Com/Tv Times
SUNDAY MORNING GRID 5/1/16 LATIMES.COM/TV TIMES 7 am 7:30 8 am 8:30 9 am 9:30 10 am 10:30 11 am 11:30 12 pm 12:30 2 CBS CBS News Sunday Face the Nation (N) Paid Program Boss Paid Program PGA Tour Golf 4 NBC News (N) Å Meet the Press (N) Å News Rescue Red Bull Signature Series (Taped) Å Hockey: Blues at Stars 5 CW News (N) Å News (N) Å In Touch Paid Program 7 ABC News (N) Å This Week News (N) NBA Basketball First Round: Teams TBA. (N) Basketball 9 KCAL News (N) Joel Osteen Schuller Pastor Mike Woodlands Amazing Paid Program 11 FOX In Touch Paid Fox News Sunday Midday Prerace NASCAR Racing Sprint Cup Series: GEICO 500. (N) 13 MyNet Paid Program A History of Violence (R) 18 KSCI Paid Hormones Church Faith Paid Program 22 KWHY Local Local Local Local Local Local Local Local Local Local Local Local 24 KVCR Landscapes Painting Joy of Paint Wyland’s Paint This Painting Kitchen Mexico Martha Pépin Baking Simply Ming 28 KCET Wunderkind 1001 Nights Bug Bites Space Edisons Biz Kid$ Celtic Thunder Legacy (TVG) Å Soulful Symphony 30 ION Jeremiah Youssef In Touch Leverage Å Leverage Å Leverage Å Leverage Å 34 KMEX Conexión En contacto Paid Program Fútbol Central (N) Fútbol Mexicano Primera División: Toluca vs Azul República Deportiva (N) 40 KTBN Walk in the Win Walk Prince Carpenter Schuller In Touch PowerPoint It Is Written Pathway Super Kelinda Jesse 46 KFTR Paid Program Formula One Racing Russian Grand Prix. -

Mammals of Jordan
© Biologiezentrum Linz/Austria; download unter www.biologiezentrum.at Mammals of Jordan Z. AMR, M. ABU BAKER & L. RIFAI Abstract: A total of 78 species of mammals belonging to seven orders (Insectivora, Chiroptera, Carni- vora, Hyracoidea, Artiodactyla, Lagomorpha and Rodentia) have been recorded from Jordan. Bats and rodents represent the highest diversity of recorded species. Notes on systematics and ecology for the re- corded species were given. Key words: Mammals, Jordan, ecology, systematics, zoogeography, arid environment. Introduction In this account we list the surviving mammals of Jordan, including some reintro- The mammalian diversity of Jordan is duced species. remarkable considering its location at the meeting point of three different faunal ele- Table 1: Summary to the mammalian taxa occurring ments; the African, Oriental and Palaearc- in Jordan tic. This diversity is a combination of these Order No. of Families No. of Species elements in addition to the occurrence of Insectivora 2 5 few endemic forms. Jordan's location result- Chiroptera 8 24 ed in a huge faunal diversity compared to Carnivora 5 16 the surrounding countries. It shelters a huge Hyracoidea >1 1 assembly of mammals of different zoogeo- Artiodactyla 2 5 graphical affinities. Most remarkably, Jordan Lagomorpha 1 1 represents biogeographic boundaries for the Rodentia 7 26 extreme distribution limit of several African Total 26 78 (e.g. Procavia capensis and Rousettus aegypti- acus) and Palaearctic mammals (e. g. Eri- Order Insectivora naceus concolor, Sciurus anomalus, Apodemus Order Insectivora contains the most mystacinus, Lutra lutra and Meles meles). primitive placental mammals. A pointed snout and a small brain case characterises Our knowledge on the diversity and members of this order. -

5-Year Review Short Form Summary
5-Year Review Short Form Summary Species Reviewed: Preble’s meadow jumping mouse (Zapus hudsonius preblei) FR Notice Announcing Initiation of This Review: March 31, 2004. 90-Day Finding for a Petition to Delist the Preble’s Meadow Jumping Mouse in Colorado and Wyoming and Initiation of a 5-Year Review (69 FR 16944-16946). Lead Region/Field Office: Region 6, Seth Willey, Recovery Coordinator, 303-236-4257. Colorado Field Office, Susan Linner, Field Supervisor, 303-236-4773. Name of Reviewer: Peter Plage, Colorado Field Office, 303-236-4750. Cooperating Field Office: Wyoming Field Office, Brian Kelly, Field Supervisor, 307-772-2374. Current Classification: Threatened rangewide. Current Recovery Priority Number: 9c. This recovery priority number is indicative of a subspecies facing a moderate degree of threat, a high recovery potential, and whose recovery may be in conflict with construction or other development projects or other forms of economic activity. Methodology used to complete the review: The 5-year review for the Preble’s meadow jumping mouse (Preble’s) was accomplished through the petition and rulemaking process. On December 23, 2003, we received two nearly identical petitions from the State of Wyoming’s Office of the Governor and from Coloradans for Water Conservation and Development, seeking to remove the Preble’s from the Federal List of Endangered and Threatened Wildlife. Both petitions were similar and maintained that the Preble’s should be delisted based on the taxonomic revision, and based on new distribution, abundance, and trends data that suggested the Preble’s was no longer threatened. On March 31, 2004, we published a notice announcing a 90-day finding that the petitions presented substantial information indicating that the petitioned action may be warranted and initiated a 5-year review (69 FR 16944-16946). -

Controlled Animals
Environment and Sustainable Resource Development Fish and Wildlife Policy Division Controlled Animals Wildlife Regulation, Schedule 5, Part 1-4: Controlled Animals Subject to the Wildlife Act, a person must not be in possession of a wildlife or controlled animal unless authorized by a permit to do so, the animal was lawfully acquired, was lawfully exported from a jurisdiction outside of Alberta and was lawfully imported into Alberta. NOTES: 1 Animals listed in this Schedule, as a general rule, are described in the left hand column by reference to common or descriptive names and in the right hand column by reference to scientific names. But, in the event of any conflict as to the kind of animals that are listed, a scientific name in the right hand column prevails over the corresponding common or descriptive name in the left hand column. 2 Also included in this Schedule is any animal that is the hybrid offspring resulting from the crossing, whether before or after the commencement of this Schedule, of 2 animals at least one of which is or was an animal of a kind that is a controlled animal by virtue of this Schedule. 3 This Schedule excludes all wildlife animals, and therefore if a wildlife animal would, but for this Note, be included in this Schedule, it is hereby excluded from being a controlled animal. Part 1 Mammals (Class Mammalia) 1. AMERICAN OPOSSUMS (Family Didelphidae) Virginia Opossum Didelphis virginiana 2. SHREWS (Family Soricidae) Long-tailed Shrews Genus Sorex Arboreal Brown-toothed Shrew Episoriculus macrurus North American Least Shrew Cryptotis parva Old World Water Shrews Genus Neomys Ussuri White-toothed Shrew Crocidura lasiura Greater White-toothed Shrew Crocidura russula Siberian Shrew Crocidura sibirica Piebald Shrew Diplomesodon pulchellum 3. -

BEFORE the SECRETARY of the INTERIOR Petition to List the Preble's Meadow Jumping Mouse (Zapus Hudsonius Preblei) As a Distinc
BEFORE THE SECRETARY OF THE INTERIOR Petition to List the Preble’s Meadow Jumping Mouse (Zapus hudsonius preblei) as a Distinct Population Segment under the Endangered Species Act November 9, 2017 Petitioners: Center for Biological Diversity Rocky Mountain Wild Acknowledgment: Conservation Intern Shane O’Neal substantially contributed to drafting of this petition. November 9, 2017 Mr. Ryan Zinke CC: Ms. Noreen Walsh Secretary of the Interior Mountain-Prairie Regional Director Department of the Interior U.S. Fish and Wildlife Service 18th and C Street, N.W. 134 Union Boulevard, Suite 650 Washington, D.C. 20240 Lakewood, CO 80228 [email protected] Dear Mr. Zinke, Pursuant to Section 4(b) of the Endangered Species Act (“ESA”), 16 U.S.C. §1533(b), Section 553(3) of the Administrative Procedures Act, 5 U.S.C. § 553(e), and 50 C.F.R. §424.14(a), the Center for Biological Diversity and Rocky Mountain Wild hereby formally petitions the Secretary of the Interior, through the United States Fish and Wildlife Service (“FWS”, “the Service”) to list the Preble’s meadow jumping mouse (Zapus hudsonius preblei) as a distinct population segment. Although the Preble’s meadow jumping mouse is already currently listed as a subspecies, this petition is necessary because of a petition seeking to de-list the Preble’s meadow jumping mouse (“jumping mouse”, “Preble’s”), filed by the Pacific Legal Foundation on behalf of their clients (PLF 2017), arguing that the jumping mouse no longer qualifies as a subspecies. Should FWS find this petition warrants further consideration (e.g. a positive 90-day finding), we are submitting this petition to ensure that the agency simultaneously considers listing the Preble’s as a distinct population segment of the meadow jumping mouse. -

Current Status of the Mammals of Balochistan Author(S)
Pakistan J. Zool., vol. 39(2), pp. 117-122, 2007. Current Status of the Mammals of Balochistan SYED ALI GHALIB, ABDUL JABBAR, ABDUR RAZZAQ KHAN AND AFSHEEN ZEHRA Department of Zoology (Wildlife and Fisheries),University of Karachi, Karachi (SAG, AZ), Forest and Wildlife Department, Government of Balochistan, Uthal (AJ) and Halcrow Pakistan (Pvt) Ltd, Karachi (ARK) Abstract.- Ninety species of mammals of Balochistan have been recorded so far belonging to 9 orders and 27 families; of these, 2lspecies are threatened,4species are endemic to Balochistan, 14 species are of special conservation interest,8 sites are important for mammals. Special efforts are being made to conserve the important mammals particularly in the protected areas specially in Chiltan Hazarganji National Park and the Hingol National Park. Key words: Biodiversity, threatened species, Balochistan, protected areas. INTRODUCTION 0030-9923/2007/0002-0117 $ 8.00/0 Copyright 2007 Zoological Society of Pakistan. al. (2002), Shafiq and Barkati (2002), Khan et al. (2004), Javed and Azam (2005), Khan and Siddiqui Balochistan is the largest province of (2005), Roberts (2005) and Roberts (2005a). Pakistan extending over an area of 350,000 sq.km As many as 2 National Parks, 14 Wildlife and the smallest number of inhabitants about 0.7 Sanctuaries and 8 Game Reserves have been million only. The province lies between 24°32’N established in the Province (Table I).At present, and 60°70’E.The-coast line is about 770 km long. detailed baseline studies on the biodiversity of The east-central and northern part of the province Hingol National Park are being undertaken under has high mountains of which considerable parts the GEF funded project on the Management of reach an elevation of above 2,300 m (7000feet) and Hingol National Park w.e.f. -

Download Season 5 Grimm Free Watch Grimm Season 5 Episode 1 Online
download season 5 grimm free Watch Grimm Season 5 Episode 1 Online. On Grimm Season 5 Episode 1, Nick struggles with how to move forward after the beheading of his mother, the death of Juliette and Adalind being pregnant. Watch Similar Shows FREE Amazon Watch Now iTunes Watch Now Vudu Watch Now YouTube Purchase Watch Now Google Play Watch Now NBC Watch Now Verizon On Demand Watch Now. When you watch Grimm Season 5 Episode 1 online, the action picks up right where the season finale of Grimm Season 4 left off, with Juliette dead in Nick's arms, Nick's mother's severed head in a box, and Agent Chavez's goons coming to kidnap Trubel. Nick is apparently drugged, and when he awakes, there is no sign that any of this occurred: no body, no box, no head, no Trubel. Was it all a truly horrible dream? Or part of a larger conspiracy? When he tries to tell his friends what happened, they hesitate to believe his claims, especially when he lays the blame at the feet of Agent Chavez. As his mental state deteriorates, will Nick be able to discover the truth about what happened and why? Where is Trubel now, and why did Chavez and her people come to kidnap her? Will his friends ever believe him, or will they continue to doubt his claims about what happened? And what will Nick do now that his and Adalind's baby is on the way? Tune in and watch Grimm Season 5 Episode 1, "The Grimm Identity," online right here at TV Fanatic to find out what happens.