Toxicological Profile for Antimony and Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Critical Mineral - Antimony
Critical Mineral - Antimony Listed as one of the 35 critical minerals by the U.S. Government, antimony strengthens metal in munitions, is used in batteries, solar panels, and wind turbines, and therefore plays an important role in our defense and energy industries. Today, China and Russia control the world’s supply of antimony, leaving the U.S. without a direct source of this mineral which is important to our national, economic, and environmental future. Perpetua Resources is developing the only commercially viable antimony mine in the U.S. to source supply for new battery and solar technologies, as well as alloys critical for national security products. CRITICAL TO THE GREEN ECONOMY Critical minerals are vital to national and economic security, as well as the future green economy. Domestic sourcing and production of all critical minerals has bi-partisan political support. Antimony is critical in the use of bearings for wind turbines, glass clarifcation for solar energy projects, and cable sheathing for wiring and as an important component in many electrical and solid state circuitry components. For defense, antimony use is critical in fame retardants, primers, and ammunition. Antimony has current and projected widespread use for the U.S. green energy sectors. Recent studies point to antimony playing a substantial role in the development of large-scale, safe, affordable battery storage technology. A limited supply of antimony and lack of advanced new antimony development projects are considered key risks to this technology moving forward. SUPPLY Currently, 92% antimony Based on the 2020 Feasibility Study the Stibnite Gold production is dominated by Project is expected to produce enough antimony to supply approximately 30% of U.S. -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Selection of Processing Tomato Genotypes Resistant to Two Spotted Spider Mite
Scientific communication VALADARES, RN; MELO, RA; SARINHO, IVF; OLIVEIRA, NS; ROCHA, FAT; SILVA, JW; MENEZES, D. 2018. Genetic diversity in accessions of melon belonging to momordica group. Horticultura Brasileira 36: 253258. DOI: http://dx.doi.org/10.1590/S0102-053620180218 Genetic diversity in accessions of melon belonging to momordica group Ricardo N Valadares1; Roberto A Melo1; Isabel VF Sarinho1; Natália S Oliveira2; Fernando AT Rocha1; José W Silva1; Dimas Menezes1 1Universidade Federal Rural de Pernambuco (UFRPE), Recife-PE, Brazil; [email protected] (corresponding author); [email protected]; [email protected]; [email protected]; [email protected]; dimasmenezes@ superig.com.b; 2Universidade Federal de Lavras (UFLA), Lavras-MG, Brazil; [email protected] ABSTRACT RESUMO The genetic divergence of melon genotypes belonging to Divergência genética em acessos de melão do grupo momordica group, collected in five Brazilian States, was estimated, momordica and the relative contribution of the morphological characters was A divergência genética de genótipos de melão do grupo determined for the genetic variability. The experimental design was momordica foi estimada, coletados em cinco estados brasileiros, randomized blocks, with four replicates. We evaluated 19 accessions e determinada a contribuição relativa dos caracteres morfológicos of melon, momordica group, two accessions of cantaloupensis group avaliados para a variabilidade genética. Foi adotado o delineamento and two commercial cultivars of inodorus group. These genotypes de blocos casualizados com quatro repetições. Nesse estudo, were characterized by 42 morphological descriptors. The data were foram utilizados 19 acessos de melão do grupo momordica, dois submitted to Tocher and UPGMA grouping methods using the genetic acessos do grupo cantaloupensis e duas cultivares comerciais do dissimilarity matrix, using Mahalanobis’ distance. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

Antimony Pentachloride Apc
ANTIMONY PENTACHLORIDE APC CAUTIONARY RESPONSE INFORMATION 4. FIRE HAZARDS 7. SHIPPING INFORMATION 4.1 Flash Point: 7.1 Grades of Purity: 99+% Common Synonyms Liquid Colorless to brown Unpleasant odor Not flammable 7.2 Storage Temperature: Ambient Antimony (V) chloride 4.2 Flammable Limits in Air: Not flammable Antimony perchloride 7.3 Inert Atmosphere: No requirement 4.3 Fire Extinguishing Agents: Not pertinent Sinks in water. Irritating vapor is produced. Freezing point is 37°F. 7.4 Venting: Pressure-vacuum 4.4 Fire Extinguishing Agents Not to Be 7.5 IMO Pollution Category: Currently not available Avoid contact with liquid. Keep people away. Used: Do not use water or foam on Wear rubber overclothing (including gloves). adjacent fires. 7.6 Ship Type: Currently not available Stop discharge if possible. 4.5 Special Hazards of Combustion 7.7 Barge Hull Type: Currently not available Evacuate. Products: Not pertinent Isolate and remove discharged material. 4.6 Behavior in Fire: Irritating fumes of Notify local health and pollution control agencies. 8. HAZARD CLASSIFICATIONS hydrogen chloride given off when water Protect water intakes. or foam is used to extinguish adjacent 8.1 49 CFR Category: Corrosive material fire. 8.2 49 CFR Class: 8 Not flammable. 4.7 Auto Ignition Temperature: Not pertinent Fire POISONOUS GASES ARE PRODUCED WHEN HEATED. 8.3 49 CFR Package Group: II DO NOT USE WATER ON ADJACENT FIRES. 4.8 Electrical Hazards: Not pertinent 8.4 Marine Pollutant: No 4.9 Burning Rate: Not pertinent 8.5 NFPA Hazard Classification: Exposure Call for medical aid. 4.10 Adiabatic Flame Temperature: Not pertinent Category Classification VAPOR Health Hazard (Blue)......... -

Generation of Carbon Dioxide and Mobilization of Antimony Trioxide by Fungal Decomposition of Building Materials John D
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School 3-25-2005 Generation of Carbon Dioxide and Mobilization of Antimony Trioxide by Fungal Decomposition of Building Materials John D. Krause University of South Florida Follow this and additional works at: https://scholarcommons.usf.edu/etd Part of the American Studies Commons Scholar Commons Citation Krause, John D., "Generation of Carbon Dioxide and Mobilization of Antimony Trioxide by Fungal Decomposition of Building Materials" (2005). Graduate Theses and Dissertations. https://scholarcommons.usf.edu/etd/730 This Dissertation is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Generation of Carbon Dioxide and Mobilization of Antimony Trioxide by Fungal Decomposition of Building Materials by John D. Krause A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Environmental and Occupational Health College of Public Health University of South Florida Major Professor: Yehia Y. Hammad, Sc.D. Noreen D. Poor, Ph.D. Ann C. Debaldo, Ph.D. Diane Te Strake, Ph.D. Date of Approval: March 25, 2005 Keywords: mold, mould, carbon dioxide, antimony trioxide, flame retardant © Copyright 2005, John D. Krause Dedication For their love, support, patience and understanding throughout this endeavor, I dedicate this work to my family, daughter, and most of all, my loving wife. Acknowledgements I would like to acknowledge the following individuals and companies for their assistance in this research. -

EROSION and BASIN MODIFICATION of SMALLER COMPLEX CRATERS in the ISIDIS REGION, MARS. J.A. Mclaughlin1 and A.K. Davatzes2, 1Temp
Lunar and Planetary Science XLVIII (2017) 1190.pdf EROSION AND BASIN MODIFICATION OF SMALLER COMPLEX CRATERS IN THE ISIDIS REGION, MARS. J.A. McLaughlin1 and A.K. Davatzes2, 1Temple University, [email protected], 2Temple University, [email protected]. Introduction: Crater erosion and morphology are Level of Degradation (LOD) was defined for each indicators of the climatic and erosional history of the crater using depth to diameter ratios (d/D) and percent- area in which the crater is found [1][2]. Here we pre- age of crater rim remaining. This categorizes craters sent a detailed analysis of crater erosion and morphol- into four types; 1 is the least degraded and 4 is the ogy in the Isidis Planitia Basin region. The goal of this most degraded. Criteria are based on the following: work is to produce a thorough database of crater prop- erties and document the modification processes that dominate locally and regionally. Crater degradation, age, and geomorphology are all considered and cross- correlated to narrow down the timing and dominance of persistent fluvial, lacustrine, and volcanic processes Floor features are complex and variable, but struc- on Mars. These geologic processes modify the crater tures indicative of the following processes were identi- rims and floor, often in distinguishable ways that pro- fied: Volcanic, Aeolian, Impact, Glacial, Mass Wast- vide insight into past environmental conditions. These ing, and Water Interaction. When possible, percent environments may hold clues to past habitable envi- floor cover of features such as lava flows, mass wast- ronments that are of particular interest for future mis- ing, dune and dust coverage were documented. -

Gsaüiiveiwibte
'C or GSAÜIIVEIWIBTE CUflIIITIIIi MïtiTHI AJIAlfïïf ,7 -' y/ . •'• .'7. 's -i, . \ STELLINGEN BEHORENDE BIJ HEJ FROEFSCHRIffT VAN R. FURLER 1. De episoomtheorie over het ontstaan van het mitochon- drion is weinig plausibel. R.A, Ratt and H.R. Mahler, Science 221 O972),575 2, Doordat S. Cirendini et al. de dragergassnelheid aan 'aet einde van een chromatografische kolom gebruiken, ontstaat een geflatteerd beeld van de weergegeven re- sultaten. Tevens is het niet mogelijk een dragergas- snelheid te berekenen zonder dat men de interstitiële porositeit kent» S, Cirendini, J. Vermont, J.C. Gressin and CL. Guilleain , J. Chromat. 84 (1973),24 3. De in de mode zijnde bepaling van RNA-moleculair ge- wichten door metingen aan formaldehyde behandelde RNA's berust op dubieuze aannamen. J.M. Kaper and M.E. v/aterworth,Virology 51 (1973),183 T.O. Diener and D.R. Smith, Virology *£ (^973), 359 M.M. El Manna and G. Bruening, Virology 56 (1973),198 4, Op grond van de zeer grote verschillen in stralingska- rakteristiek van de isotopen 1-131 en 1-123 is het streven van isotopenproducenten om een zo 'schoon' mogelijk 1-123 voor diagnostische doeleinden te leve- ren in strijd met de volksgezondheid, doordat de ver- tragingen#die dit oplevert onnodige stralingsbelasting voor patiënten veroorzaakt. H. ïlishiyama et al. J.Nucl.Med. 1£ (1974),261 5« De analogie die Gilbert et al. opmerken tussen de "exchange peak" in de kolom vloaistofchromatografie met behulp van ionenwisselaar en de luchtpi.ek bij gaschromatografie is twijfelachtig. T.W. Gilbert and R.A, Dobbs, Analyt.Chem. 45 (1>73), 1390. -

A Unified Plane Coordinate Reference System
This dissertation has been microfilmed exactly as received COLVOCORESSES, Alden Partridge, 1918- A UNIFIED PLANE COORDINATE REFERENCE SYSTEM. The Ohio State University, Ph.D., 1965 Geography University Microfilms, Inc., Ann Arbor, Michigan A UNIFIED PLANE COORDINATE REFERENCE SYSTEM DISSERTATION Presented in Partial Fulfillment of the Requirements for The Degree Doctor of Philosophy in the Graduate School of The Ohio State University Alden P. Colvocoresses, B.S., M.Sc. Lieutenant Colonel, Corps of Engineers United States Army * * * * * The Ohio State University 1965 Approved by Adviser Department of Geodetic Science PREFACE This dissertation was prepared while the author was pursuing graduate studies at The Ohio State University. Although attending school under order of the United States Army, the views and opinions expressed herein represent solely those of the writer. A list of individuals and agencies contributing to this paper is presented as Appendix B. The author is particularly indebted to two organizations, The Ohio State University and the Army Map Service. Without the combined facilities of these two organizations the preparation of this paper could not have been accomplished. Dr. Ivan Mueller of the Geodetic Science Department of The Ohio State University served as adviser and provided essential guidance and counsel. ii VITA September 23, 1918 Born - Humboldt, Arizona 1941 oo.oo.o BoS. in Mining Engineering, University of Arizona 1941-1945 .... Military Service, European Theatre 1946-1950 o . o Mining Engineer, Magma Copper -
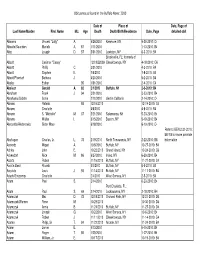
Obituaries Buffalo News 2010 by Name
Obituaries as found in the Buffalo News: 2010 Date of Place of Date, Page of Last Name/Maiden First Name M.I. Age Death Death/Birth/Residence Date, Page detailed obit Abbarno Vincent "Lolly" A. 9/26/2010 Kenmore, NY 9-30-2010: C4 Abbatte/Saunders Murielle A. 87 1/11/2010 1-13-2010: B4 Abbo Joseph D. 57 5/31/2010 Lewiston, NY 6-3-2010: B4 Brooksville, FL; formerly of Abbott Casimer "Casey" 12/19/22009 Cheektowaga, NY 4-18-2010: C6 Abbott Phillip C. 3/31/2010 4-3-2010: B4 Abbott Stephen E. 7/6/2010 7-8-2010: B4 Abbott/Pfoetsch Barbara J. 4/20/2010 5-2-2010: B4 Abeles Esther 95 1/31/2010 2-4-2010: C4 Abelson Gerald A. 82 2/1/2010 Buffalo, NY 2-3-2010: B4 Abraham Frank J. 94 3/21/2010 3-23-2010: B4 Abrahams/Gichtin Sonia 2/10/2010 died in California 2-14-2010: C4 Abramo Rafeala 93 12/16/2010 12-19-2010: C4 Abrams Charlotte 4/6/2010 4-8-2010: B4 Abrams S. "Michelle" M. 37 5/21/2010 Salamanca, NY 5-23-2010: B4 Abrams Walter I. 5/15/2010 Basom, NY 5-19-2010: B4 Abrosette/Aksterowicz Sister Mary 6/18/2010 6-19-2010: C4 Refer to BEN 2-21-2010: B6/7/8 for more possible Abshagen Charles, Jr. L. 73 2/19/2010 North Tonawanda, NY 2-22-2010: B8 information Acevedo Miguel A. 10/6/2010 Buffalo, NY 10-27-2010: B4 Achkar John E. -

4. Inorganic Flame Retardants. Plastics Can Be Given Flame Retardant Characteristics by Introducing Elements of Organic, Inorganic and Halogen Origin
4. Inorganic Flame Retardants. Plastics can be given flame retardant characteristics by introducing elements of organic, inorganic and halogen origin. Such elements include magnesium, aluminium, phosphorous, molybdenum, antimony, tin, chlorine and bromine. Flame retardants are added in either the manufacturing step of the polymer or the compounding step of the polymeric article. Phosphorous bromine and chlorine are usually included as some organic compound. Inorganic flame retardants are usually added together with other flame retardants to provide a more efficient flame retardant action through synergism. Halogen flame retardants usually need an addition of about 40% in order to be effective, and this affects the properties of the polymer quite negatively. Structural integrity of the polymer article is often very important, and a drastic decrease in strength and other mechanical properties is simply not acceptable. The efficiency of halogen flame retardants is often enhanced by the addition of inorganic flame retardants. A smaller mass percentage halogen flame retardant is now needed, so the adverse effect on the polymer properties is also reduced (Touval, 1993) . 4.1 Antimony Compounds The antimony compounds used for flame retardancy include antimony trioxide, antimony pentoxide and antimony-metal compounds. In 1990 in the United States alone, the use of antimony trioxide amounted to 20 000 metric tons just for the flame retardancy of plastics. Antimony oxide is readily found in nature but in very impure form. This is not suitable for 29 direct use as flame retardant, so antimony oxide is often rather produced from antimony metal. There are therefore many different grades of antimony oxide that can be used for flame retardants. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime.