Baseline Report Series: 3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2 Shipley Cottages Bridgnorth Road, Pattingham, WV6 7EH
2 Shipley Cottages Bridgnorth Road, Pattingham, WV6 7EH 2 Shipley Cottages Bridgnorth – 7.9 miles Wolverhampton – 6.3 miles (Distances are approximate) A pleasantly spacious, 3-bedroom semi- detached property in an enviable rural position, with wonderful views across the surrounding countryside. Very convenient distance from Wolverhampton, Bridgnorth, Telford, and local travel links. Entrance Hall Lounge Dining Room Breakfast Kitchen Utility Room Master Bedroom Guest Bedroom Third Bedroom Family Bathroom Gated Driveway Parking Sizeable Outbuilding With Power Ample Gardens to Front & Rear Wonderful Countryside Views 2 Shipley Cottages This spacious 3-bedroom semi-detached house boasts an enviable rural location, with views across open countryside and easy access to local amenities and travel links. Having two comfortable reception rooms, both with fireplaces, the property also benefits from a sizeable breakfast kitchen and a useful utility. Two double bedrooms and one ample single can be found across the first floor, alongside a well-appointed bathroom. The property overlooks open fields and woodland, with wonderful rural views to the east stretching as far as Wolverhampton and Pattingham village. Set behind a gated driveway with gardens to the front and rear, the property includes a large outbuilding with power supply, additional kennels and outdoor storage. A timber door opens to the hall, which is of a good size with half-glazed timber white. A cupboard here conceals the hot-water tank and storage. The master surrounded by the open countryside which separates these two distinct and doors off and attractive wood-effect flooring, continuing in to both reception bedroom is located at the front of the property, an excellent double with a diverse conurbations. -

Things to Do and Places to Go Sept 2020
Things To Do And Places to Go! September 2020 Table of Contents Parks and Nature Reserves ............................................................................................... 3 Arrow Valley Country Park ....................................................................................................... 3 Clent Hills ................................................................................................................................ 3 Cofton Park .............................................................................................................................. 3 Cannon Hill Park ...................................................................................................................... 3 Highbury Park .......................................................................................................................... 3 King’s Heath Park ..................................................................................................................... 4 Lickey Hills ............................................................................................................................... 4 Manor Farm ............................................................................................................................. 4 Martineau Gardens .................................................................................................................. 4 Morton Stanley Park ............................................................................................................... -

1 the Courtyard, Back Lane, Ackleton, Worfield, Shropshire
1 The Courtyard, Back Lane, Ackleton, Worfield, Shropshire, WV6 7JJ 1 The Courtyard, Back Lane, Ackleton, Worfield, Shropshire, WV6 7JJ A delightful barn conversion standing in an attractive courtyard development in an exceptionally sought after Shropshire village which is within easy reach of Wolverhampton, Bridgnorth and Telford. LOCATION and filtration unit above, plumbing for a dishwasher, plumbing for a washing machine, an integrated The Courtyard stands in a lovely position within a sought after village which is renowned for The Red fridge, stainless steel sink, tiled floor, a beamed and raftered ceiling and a double glazed rear Cow public house / restaurant. Local facilities are readily available in Albrighton, which is within a few window and door to the garden. minutes drive, with a wide range of shops and a train station with direct services to Birmingham. A staircase with turn balustrading rises from the hall to the galleried first floor landing with a double Wolverhampton, Telford and Bridgnorth are easily accessible, the M54 can be reached at either glazed window, ceiling beam and linen cupboard with slatted shelving and radiator. The PRINCIPAL Junction 3 or 4 and there is a wide range of schools in both sectors within convenient travelling SUITE has a double bedroom with a light corner aspect with double glazed windows to two distance. elevations, exposed ceiling timbering and an EN-SUITE SHOWER ROOM with fully tiled shower cubicle, WC with concealed flush and wash basin with cupboards beneath, tiled floor, part tiled walls, DESCRIPTION a double glazed window and a towel rail radiator. BEDROOM TWO is a double room in size with 1 The Courtyard is a well proportioned barn conversion which forms part of an exclusive courtyard exposed ceiling timbering, access to the roof space and a double glazed window. -

Notes from the Bridgnorth, Worfield, Alveley, Claverley and Brown Clee Local Joint Committee Meeting Held on Wednesday 13Th Apri
Committee and Date Item No Bridgnorth, Worfield, Alveley, Claverley and Brown Clee Local Joint Committee A Wednesday 13th April Public 2016 NOTES FROM THE BRIDGNORTH, WORFIELD, ALVELEY, CLAVERLEY AND BROWN CLEE LOCAL JOINT COMMITTEE MEETING HELD ON WEDNESDAY 13TH APRIL 2016 AT 7:00PM AT THE PEOPLE’S HALL EVANGELICAL CHURCH, ST JOHN STREET, BRIDGNORTH. (7.00 – 9.15 p.m.) Responsible Tracy Johnson Officer email: [email protected] Tel: 07990 085122 Committee Members Present: Shropshire Council Christian Lea John Hurst-Knight Tina Woodward Les Winwood Town/Parish Councils Sue Morris, Astley Abbotts Parish Council David Cooper, Bridgnorth Town Council Peter Dent, Tasley Parish Council Len Ball, Worfield and Rudge Parish Council West Mercia Police Sgt Sarah Knight CSO Sue Eden Shropshire Fire and Rescue Service Fire Officer Ashley Brown 1 1. Welcome, Introductions and Apologies for absence ACTION Cllr Christian Lea welcomed everyone to the People’s Hall Evangelical Church, Bridgnorth. Apologies were received on behalf of the following: - Cllr William Parr, Shropshire Council, Cllr Michael Wood, Shropshire Council, Sgt Bailey – West Mercia Police. 2. Declaration of interest None 3. To consider and approve the notes of the meeting held on 6th October 2015 (Attached marked ‘A’) The notes were agreed and signed by the Chair. 4. Community Safety West Mercia Constabulary 4.1 Sgt Sarah Knight introduced herself has she is taking over from Sgt Richard Bailey 4.2 Sgt Sarah Knight stated if you want to know more about crime in your area even down to street level to use the following website: http://www.police.uk/ 4.3 Currently West Mercia Police are running two crime schemes the first one is “We don’t buy Crime” campaign this will be spear headed by 1,400 smartwater kits that were delivered to households in Cleobury Mortimer which has proved to be a success. -

Notice of Poll Bromsgrove 2021
NOTICE OF POLL Bromsgrove District Council Election of a County Councillor for Alvechurch Electoral Division Notice is hereby given that: 1. A poll for the election of a County Councillor for Alvechurch Electoral Division will be held on Thursday 6 May 2021, between the hours of 07:00 am and 10:00 pm. 2. The number of County Councillors to be elected is one. 3. The names, home addresses and descriptions of the Candidates remaining validly nominated for election and the names of all persons signing the Candidates nomination paper are as follows: Names of Signatories Name of Candidate Home Address Description (if any) Proposers(+), Seconders(++) & Assentors BAILES 397 Birmingham Road, Independent Kilbride Karen M(+) Van Der Plank Alan Bordesley, Redditch, Kathryn(++) Worcestershire, B97 6RH LUCKMAN 40 Mearse Lane, Barnt The Conservative Party Woolridge Henry W(+) Bromage Daniel P(++) Aled Rhys Green, B45 8HL Candidate NICHOLLS 3 Waseley Road, Labour Party Hemingway Oreilly Brett A(++) Simon John Rubery, B45 9TH John L F(+) WHITE (Address in Green Party Ball John R(+) Morgan Kerry A(++) Kevin Bromsgrove) 4. The situation of Polling Stations and the description of persons entitled to vote thereat are as follows: Station Ranges of electoral register numbers of Situation of Polling Station Number persons entitled to vote thereat Rowney Green Peace Mem. Hall, Rowney Green Lane, Rowney 1 ALA-1 to ALA-752 Green Beoley Village Hall, Holt End, Beoley 2 ALB-1 to ALB-809 Alvechurch Baptist Church, Red Lion Street, Alvechurch 3 ALC-1 to ALC-756 Alvechurch -

Minute Book for the Manor of Wyken Beginning 15Th March 1785 Ending 1850 (Shropshire Archives
Minute Book for the Manor of Wyken beginning 15th March 1785 ending 1850 (Shropshire Archives. 1190/1/448 & /449) The Court Baron purchased of William Yelverton Davenport held at the Wheel Inn 1785 before James Marshall Steward and in the presence of John Bache gentleman and John Nicholas, blacksmith. Allan Pollock of Chiselhurst, Kent, Doctor of Physick demise [lease] of Lord’s Meadow late Bache’s and one messuage and one yard land to Charles Bowen of Catstrey for 21 years or as Pollock sees fit. Pollock pays a fine of 1s for Lord’s Meadow and for the remainder (except the16th part of a nook or a small yard) 9s 9d Court Baron 23 July held at the house of Thomasine Painton, widow, known as the Wheel Inn Steward etc as above Thomas Smith of Hilton produces a letter of attorney for Job Beard of Halfway House, Quatford, innkeeper (16th part of a nook and a small yard). In 1767 it was occupied by Mary Smith spinster and Elizabeth Smith widow. Mortgaged to Job Beard for £36. Received from William Taylor £36 and the property goes to the work and use of Elizabeth Taylor wife of William Taylor, her heirs and assigns. Fine at ingoing 3 3/4d. Elizabeth Taylor then surrenders a 32nd part of a nook ie an eighth of an acre and half of the little yard to the use of Elizabeth Hill, widow and her heirs and assigns. Fine 2d. [a nook is therefore 4 acres] 27th March 1786 Steward etc as above John Rowley, maltster, of Ackleton and Elizabeth his wife (she examined separately) surrender into the hands of the lord a cottage and garden (formerly Nicholas’s) in Worfield to the use of William Slater of Winscote, his heirs and assigns. -

Green Infrastructure Framework 3: Access and Recreation
Planning for a Multifunctional Green Infrastructure Framework in Worcestershire Green Infrastructure Framework 3: Access and Recreation May 2013 Find out more online: www.worcestershire.gov.uk/ Contents Contents 1 Chapter 1: Introduction 2 Chapter 2: Context 4 Chapter 3: Informal Recreation Provision in Worcestershire 6 Chapter 4: Carrying Capacity of GI Assets 16 Chapter 5: Green Infrastructure Assets and Indices of Multiple Deprivation 24 Chapter 6: Pressure from Development 38 Chapter 7: Future Needs and Opportunities 42 Chapter 8: Summary and Conclusions 53 Appendix 1: Sub-regional assets covered by the study 54 Appendix 2: Linear sub-regional GI assets 56 Appendix 3: Accessible Natural Greenspace Standard 57 Appendix 4: Proposed Housing Development Sites in the County 58 1 Chapter 1: Introduction Preparation of this Green Infrastructure Framework Document 3 Access and Recreation has been led by the County Council's Strategic Planning and Environmental Policy team. The framework has been endorsed by the Worcestershire Green Infrastructure Partnership. Partnership members include the Worcestershire Wildlife Trust, Natural England, Environment Agency, Forestry Commission, English Heritage, the County and District Councils and the Voluntary Sector. Background to the Framework The Green Infrastructure partnership is producing a series of 'framework documents' which provide the evidence base for the development of the GI Strategy. Framework Document 1 is an introduction to the concept of Green Infrastructure (GI) and also identified the need for the strategic planning of GI and the policy drivers that support the planning of GI at differing spatial scales. Framework Document 2 is an introduction to the natural environment landscape, biodiversity and historic environment datasets and developed the concept of GI Environmental Character Areas based on the quality and quantity of the natural environment assets. -

Headteacher: Mrs C Gaskin
q Worfield Endowed Church of England Primary School Main Street, Worfield, Bridgnorth, Shropshire WV15 5LF PROSPECTUS Headteacher: Mrs C Gaskin Tel: 01746 716606 Fax: 01746 716237 email: [email protected] School website: www.worfield.shropshire.sch.uk Thank you for your interest in Worfield Primary School. I hope you will find this prospectus a useful introduction to our school. Our school has a warm, vibrant atmosphere where purposeful learning takes place. The teaching and support staff are enthusiastic, hard working and caring. We aim to provide a quality education for your child and hope that you will work in partnership with us towards this. The school was opened in 1846, the building being funded with money from ancient endowments. As a Church of England aided school, there are strong links with St. Peter‟s Church, Worfield. The school provides education for boys and girls beginning at the age of 3+ in our Nursery and progressing to the age of 11. The school is situated just off the picturesque Main Street of half- timbered houses and old cottages, leading to the 12th century church and the Tudor manor house. The River Worfe runs through the school grounds and the adjoining nature trail provides an environmental resource for the pupils of this school. If you would like to find out more about the school, please telephone for an appointment. I would be pleased to show you around. Yours sincerely Mrs Claire Gaskin Headteacher 2 Staff at Worfield Endowed C of E Primary School Headteacher: Mrs C. Gaskin Deputy Headteachers: Mrs L. -

L02-2135-02B-Intervisibilty B
LEGEND Pole Bank 516m AOD (NT) Malvern Hills AONB (Note 3) Notes: Adjacent AONB boundaries LONGER DISTANCE VIEWS 1. Base taken from A-Z Road Maps for Birmingham (Note 3) and Bristol FROM BEYOND BIRMINGHAM 2. Viewpoints have been selected to be Brown Clee Hill representative, and are not definitive 540m AOD 3. Taken from www.shropshirehillsaonb.co.uk Adjacent National Park (Note 7) website, www.cotswoldaonb.com website, Malvern Kinver Edge Hills District Council Local Plan Adopted 12th July 155m AOD (NT) 2006, Forest of Dean District Local Plan Review 30km Distance from spine of Malvern Hills Adopted November 2005, Herefordshire Unitary Clent Hills 280m Development Plan Adopted 23rd March 2007 and AOD (NT) wyevalleyaonb.org.uk website 4. Observer may not nessecarily see all of Titterstone Clee 10 intervening land between viewpoint and Malvern 1 Viewpoint used as visual receptor SHROPSHIRE AONB Hill 500m AOD Hills 14 5. Information obtained from the Malvern Hill Conservators Intervisibility viewing corridor 6. Views outside inner 15km study area graded on Appendix Table 1, but not shown graded on plan L02. M5 alongside 7. Taken from OS Explorer MapOL13. Clows Top Malvern Hills High Vinnals 11 Bromsgrove 100m AOD Harley’s Mountain 231m AOD A 370m AOD 50km 386m AOD Bircher Common 160-280m AOD (NT) Hawthorn Hill 30km 407m AOD Bradnor Hill 391m AOD (NT) Hergest Ridge 426m AOD Malvern Hills (Note 4) 22 peaks including from north to south: A-End Hill 1079ft (329m) 41 Glascwn Hill Westhope B-North Hill 1303ft (397m) 522m AOD Hill 120m C-Sugarloaf -
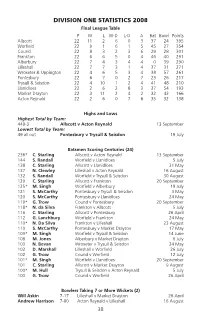
Division One Statistics 2008
DIVISION ONE STATISTICS 2008 Final League Table P W L W-D L-D A Bat Bowl Points Allscott 22 11 2 6 0 3 37 24 395 Worfield 22 9 1 6 1 5 45 27 354 Cound 22 8 3 2 3 6 29 28 301 Frankton 22 6 6 5 0 4 46 40 291 Alberbury 22 7 4 3 4 4 0 39 290 Lilleshall 22 7 7 3 1 4 37 31 271 Wroxeter & Uppington 22 4 6 5 3 4 38 57 261 Pontesbury 22 6 7 0 2 7 23 26 217 Trysull & Seisdon 22 4 10 1 2 4 41 48 210 Llanidloes 22 2 6 2 8 3 37 54 193 Market Drayton 22 3 11 2 4 2 32 43 166 Acton Reynald 22 2 6 0 7 6 35 32 138 Highs and Lows Highest Total by Team: 449-3 Allscott v Acton Reynald 13 September Lowest Total by Team: 49 all out Pontesbury v Trysull & Seisdon 19 July Batsmen Scoring Centuries (24) 236* C. Starling Allscott v Acton Reynald 13 September 144 S. Randall Worfield v Llanidloes 5 July 138 C. Starling Allscott v Llanidloes 31 May 137 N. Clewley Lilleshall v Acton Reynald 16 August 132 S. Randall Worfield v Trysull & Seisdon 30 August 129 C. Starling Allscott v Frankton 20 September 125* M. Singh Worfield v Alberbury 19 July 121 S. McCarthy Pontesbury v Trysull & Seisdon 3 May 120 S. McCarthy Pontesbury v Llanidloes 24 May 119* G. Trow Cound v Pontesbury 20 September 118* N. -

Vebraalto.Com
The Guildford Wychbury Fields Hagley DY9 0QF Price £499,950 In a picturesque setting at the foot of Clent Hills, our exclusive Wychbury Fields development of two, three, four and five bedroom homes enjoy an open outlook, with some offering views towards the Malvern Hills. Generous in space and scope, as well as boasting high specification throughout, these light and airy homes benefit from a wealth of local amenities and respected schools. While the commuting and entertaining appeal of Birmingham is only a short 15-mile drive away. Commuting from this desirable semi-rural retreat is just as convenient. Whether the office, family or school run calls, you can be there in next to no time, with access to Junction 3 of the M5 located about six miles away from Hagley taking you straight to the city centre. Or you can take advantage of the direct trains from Hagley station to Birmingham Moor Street in around 34 minutes, as well as to Worcester, Solihull and Cheltenham. Regular buses also run to Stourbridge via A491 and Kidderminster on the A456. The sought-after village of Hagley can be found just to the south of Stourbridge on the Worcestershire border. An affluent and leafy residential suburb, it’s also home to a lovely choice of independent shops, country pubs and fashionable eateries. Famous for glass production, Stourbridge offers a wealth of banks, high street stores and supermarkets including Waitrose, together with popular bars and restaurants. While Merry Hill Shopping Centre in Dudley is about seven miles away and the city buzz of Birmingham is a little further. -

19 Stone Lane, Kinver, Stourbridge, West Midlands DY7 6DZ 01562 820880
19 Stone Lane, Kinver, Stourbridge, West Midlands DY7 6DZ 01562 820880 FOR SALE Guide price £299,950 2 reception 3 bedrooms 1 Bath/Shower Rear garden rooms rooms Mileage (distances approximate) Birmingham 17 miles – Stourbridge 5 miles – Kidderminster 6 miles – Worcester 20 miles Traditional Extended Family Home The village offers an array of useful and attractive An internal inspection is recommended to appreciate the Living & Family Rooms 3 Double Bedrooms amenities including shops, restaurants and public quality and nature of this improved semi-detached family Extended Kitchen Utility Room houses as well as three schools: Foley Infant School, home. Contemporary Bathroom Cloakroom Brindley Heath Junior School and Edgecliff High South Facing Gardens Off road parking School. A tarmacadam driveway leading to the front entrance Sought after village location and a stepped canopy porch entrance into the main Viewing Essential There are 280 acres of National Trust land nearby reception hall with straight flight staircase leading to the including Kinver Edge to the south-west of the village first floor and an original solid wooden door into the front DIRECTIONS with a Country Park nearby. The views from the top of living room. From Kidderminster proceed in a Northerly direction Kinver Edge are wonderful as it is a sandstone ridge on the A449 Wolverhampton Road. Take a left turn standing several hundred feet high, with views out The LIVING ROOM has an attractive open cast iron onto Dunsley Road towards Kinver, continue over the over surrounding fields and woodland. This really is a fireplace with a tiled hearth and a double glazed bay hump back bridge onto Mill Lane and bear right onto perfect location for family and village life! window to front and fitted cupboards and shelving to the the High Street.