Plugging the Chemical World Into the Information Society
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synthetic Worlds Nature, Art and the Chemical Industry
Synthetic Worlds Nature, Art and the Chemical Industry Esther Leslie Synthetic Worlds Synthetic Worlds Nature, Art and the Chemical Industry Esther Leslie reaktion books Published by reaktion books ltd www.reaktionbooks.co.uk First published 2005 Copyright © Esther Leslie 2005 All rights reserved No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers. Colour printed by Creative Print and Design Group, Harmondsworth, Middlesex Printed and bound in Great Britain by Biddles Ltd, Kings Lynn British Library Cataloguing in Publication Data Leslie, Esther, 1964– Synthetic worlds: nature, art and the chemical industry 1.Art and science 2.Chemical industry - Social aspects 3.Nature (Aesthetics) I. Title 7-1'.05 isbn 1 86189 248 9 Contents introduction: Glints, Facets and Essence 7 one Substance and Philosophy, Coal and Poetry 25 two Eyelike Blots and Synthetic Colour 48 three Shimmer and Shine, Waste and Effort in the Exchange Economy 79 four Twinkle and Extra-terrestriality: A Utopian Interlude 95 five Class Struggle in Colour 118 six Nazi Rainbows 167 seven Abstraction and Extraction in the Third Reich 193 eight After Germany: Pollutants, Aura and Colours That Glow 218 conclusion: Nature’s Beautiful Corpse 248 References 254 Select Bibliography 270 Acknowledgements 274 Index 275 introduction Glints, Facets and Essence opposites and origins In Thomas Pynchon’s novel Gravity’s Rainbow a character remarks on an exploding missile whose approaching noise is heard only afterwards. The horror that the rocket induces is not just terror at its destructive power, but is a result of its reversal of the natural order of things. -
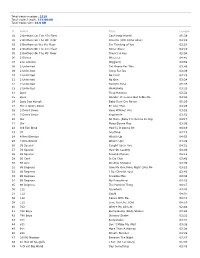
Total Tracks Number: 2218 Total Tracks Length: 151:00:00 Total Tracks Size: 16.0 GB
Total tracks number: 2218 Total tracks length: 151:00:00 Total tracks size: 16.0 GB # Artist Title Length 01 2 Brothers On The 4Th Floor Can't Help Myself 05:39 02 2 Brothers On The 4th Floor Dreams (Will Come Alive) 04:19 03 2 Brothers on the 4th Floor I'm Thinking of You 03:24 04 2 Brothers On The 4Th Floor Never Alone 04:10 05 2 Brothers On The 4th Floor There's A Key 03:54 06 2 Eivissa Oh La La 04:41 07 2 In a Room Wiggle It 03:59 08 2 Unlimited Get Ready For This 03:40 09 2 Unlimited Jump For Joy 03:39 10 2 Unlimited No Limit 03:28 11 2 Unlimited No One 03:24 12 2 Unlimited Twilight Zone 05:36 13 2 Unlimited Workaholic 03:33 14 2pac Thug Mansion 03:32 15 2pac Wonder If Heaven Got A Ghetto 04:34 16 2pac Daz Kurupt Baby Dont Cry Remix 05:20 17 Three Doors Down Be Like That 04:25 18 3 Doors Down Here Without You 03:53 19 3 Doors Down Kryptonite 03:52 20 3lw No More (Baby I'm Gonna Do Rig 04:17 21 3lw Playa Gonna Play 03:06 22 3rd Eye Blind How Is It Gonna Be 04:10 23 3T Anything 04:15 24 4 Non Blondes What's Up 04:55 25 4 Non Blonds What's Up? 04:09 26 38 Special Caught Up In You 04:25 27 38 Special Hold On Loosely 04:40 28 38 Special Second Chance 04:12 29 50 Cent In Da Club 03:42 30 50 cent Window Shopper 03:09 31 98 Degrees Give Me One More Night (Una No 03:23 32 98 Degrees I Do (Cherish You) 03:43 33 98 Degrees Invisible Man 04:38 34 98 Degrees My Everything 04:28 35 98 Degrees The Hardest Thing 04:27 36 112 Anywhere 04:03 37 112 Cupid 04:07 38 112 Dance With Me 03:41 39 112 Love You Like I Did 04:16 40 702 Where My Girls At 02:44 -

Readingsample
SpringerBriefs in Molecular Science From the Molecular World A Nineteenth-Century Science Fantasy Bearbeitet von Alan J. Rocke, Hermann Kopp 1. Auflage 2012. Taschenbuch. vii, 105 S. Paperback ISBN 978 3 642 27415 2 Format (B x L): 15,5 x 23,5 cm Gewicht: 189 g Weitere Fachgebiete > Chemie, Biowissenschaften, Agrarwissenschaften > Chemie Allgemein > Geschichte der Chemie Zu Inhaltsverzeichnis schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. From the Molecular World (Printed as Manuscript.) Printed by C. F[riedrich] Winter’s Book-Press in Darmstadt. 18821 [Contents: v Preface 1 Introduction 2 Simple Atoms with Hands 6 The Behavior of Gas Molecules 10 Friendship and Affinity 16 Two Conceptions of the Constitutions of Molecules 29 The Constitutions of Organic Substances 34 A Personal Comment 34 In the Circus 38 Radicals 42 In the Dance Club 45 Atoms of Variable Handedness 52 The Story of Iodine and Hydrogen 59 Friendship under Extreme Heat 68 Molecular States 75 The World of Liquid Molecules 80 In the Annex 90 The Dance of Electrolysis 94 The World of Hydrated Salt Solutions 105 Conclusion] 1 This title-page language represents the first (private) printing of this book. See Introduction, Sections 3 and 4, for the printing history of this book. In Kopp’s book, there are no section- heads (or any other subdivisions); those given in this translation were provided by the present editor, as was everything else that appears here in square brackets. -

Page 1 of 163 Music
Music Psychedelic Navigator 1 Acid Mother Guru Guru 1.Stonerrock Socks (10:49) 2.Bayangobi (20:24) 3.For Bunka-San (2:18) 4.Psychedelic Navigator (19:49) 5.Bo Diddley (8:41) IAO Chant from the Cosmic Inferno 2 Acid Mothers Temple 1.IAO Chant From The Cosmic Inferno (51:24) Nam Myo Ho Ren Ge Kyo 3 Acid Mothers Temple 1.Nam Myo Ho Ren Ge Kyo (1:05:15) Absolutely Freak Out (Zap Your Mind!) 4 Acid Mothers Temple & The Melting Paraiso U.F.O. 1.Star Child vs Third Bad Stone (3:49) 2.Supernal Infinite Space - Waikiki Easy Meat (19:09) 3.Grapefruit March - Virgin UFO – Let's Have A Ball - Pagan Nova (20:19) 4.Stone Stoner (16:32) 1.The Incipient Light Of The Echoes (12:15) 2.Magic Aum Rock - Mercurical Megatronic Meninx (7:39) 3.Children Of The Drab - Surfin' Paris Texas - Virgin UFO Feedback (24:35) 4.The Kiss That Took A Trip - Magic Aum Rock Again - Love Is Overborne - Fly High (19:25) Electric Heavyland 5 Acid Mothers Temple & The Melting Paraiso U.F.O. 1.Atomic Rotary Grinding God (15:43) 2.Loved And Confused (17:02) 3.Phantom Of Galactic Magnum (18:58) In C 6 Acid Mothers Temple & The Melting Paraiso U.F.O. 1.In C (20:32) 2.In E (16:31) 3.In D (19:47) Page 1 of 163 Music Last Chance Disco 7 Acoustic Ladyland 1.Iggy (1:56) 9.Thing (2:39) 2.Om Konz (5:50) 10.Of You (4:39) 3.Deckchair (4:06) 11.Nico (4:42) 4.Remember (5:45) 5.Perfect Bitch (1:58) 6.Ludwig Van Ramone (4:38) 7.High Heel Blues (2:02) 8.Trial And Error (4:47) Last 8 Agitation Free 1.Soundpool (5:54) 2.Laila II (16:58) 3.Looping IV (22:43) Malesch 9 Agitation Free 1.You Play For -

CRE 209 18 Wheeler the Revea
Artist Record Format Notes 18 Wheeler Boddha UK 7" CRE 198 18 Wheeler Steel Guitars UK 7" CRE 209 18 Wheeler The Revealer UK 7" CRE 188 60 FT Dolls Alison's Room UK 7" Red vinyl - # 2094 60 FT Dolls Happy Shopper UK 7" Singned by all three members - gray vinyl 60 FT Dolls Talk To Me UK 7" Signed by all three members - orange vinyl 60 FT Dolls The Big 3 US CS Promo Geffen promo cassette Arcadia Goodbye Is Forever US 7" Picture sleeve Arcadia So Red The Rose US LP With lyric insert Arcadia Election Day US 7" Picture sleeve Arnold Barn Tapes UK 10" Arnold Windsor Park UK 7" CRE 300 Ash Angel Interceptor UK 7" # 95 Audience, The A Pessimist Is Never Disappointed UK 7" Yellow vinyl with poster Babe The Blue Ox Chicken Head Bone Sucker US 7" Ballroom Take It UK 7" MUM 92 Ballroom Take It UK 7" MUM 106 - later release with different sleeve Beekeepers, The Hold On UK 7" Bernard Butler Stay Together UK 7" CRE 281 Bikini Kill I Like F***ing US 7" Kill Rockstars - with sticker and insert/poem Bikini Kill New Radio US 7" Kill Rockstars Bikini Kill Peel Sessions US 7" Bikini Kill/Huggy Bear Split 12" UK LP Bis Keroleen UK 7" Split 7" with Heavenly - K Records Bis Pop Song UK 7" Split 7" with Lugworm Bis Sweet Shop Avengers UK 7" Wiiija - pink sleeve Bis Sweet Shop Avengers (Hi-Fi Mix) UK 7" Wiiija - yellow sleeve Black Box Recorder Child Psychology UK 7" Bluetones Marblehead Johnson UK 7" # 1043 Bluetones Sleazy bed track UK 7" # 2504 Blur Charmless Man UK 7" FOOD 77 - Gatefold Blur Charmless Man US CD Promo Promo CD with unique sleeve Blur Chemical World UK 7" FOOD 45 - red vinyl Blur Country House UK CD Blur M.O.R. -
![714.01 [Cover] Mothernew3](https://docslib.b-cdn.net/cover/7202/714-01-cover-mothernew3-4917202.webp)
714.01 [Cover] Mothernew3
NewMusic ® CMJ 25 SUPER FURRY ANIMALS Report REVIEWED: LIFESAVAS, IMA ROBOT, VUSI MAHLASELA, CMJ SPOTLIGHT PORCUPINE TREE... PLUS MORE! Issue No. 822 • July 14, 2003 • www.cmj.com DANGER! HIGH VOLTA CMJ The Mars Volta Gets Ready To Say What Words Never Can CMJ RETAIL To Infinity And Beyonce STATION PROFILE Hearing Voices: Maine’s WERU MUSIC NEWS Cat Claws The Lips RADIO 200:RADIOHEAD LOCKED AT NO. 1 • SUPER FURRY ANIMALS MOST ADDED 7/14/2003 Issue No. 822 • Vol 76 • No. 7 COVER STORY 8 Escape From Mars While Mars Volta’s debut LP, De-Loused In The Comatorium, wrings arrhythmic chaos, intricate prog-rock and shrill harmonies, Mars Volta itself was wrought with bereavement after the loss of keyboardist and partner-in-arms Jeremy Ward, who died mid-tour. Guitarist Omar Rodriguez- Lopez checks in with CMJ to discuss the redemptive qualities of playing music; something the group will need as the remaining members attempt to pick up the pieces and carry on. DEPARTMENTS 4 The Week In Industry News or not, the Animal’s sixth record is sure to The RIAA unleashes its lawyers on the unsus- blow listeners away with its surreal, epic pop. 8 pecting public; Major League Baseball hits a grand slam with Clear Channel. 24 Tours Liz Phair shakes up Tour Of The Week, 5 The Week In Music News Beautiful Mistake leads the Vital Five, and The Flaming Lips get into a Cat-fight; Open Hand, Lonesome Organist, Stephan Spiritualized finds Grace with Sanctuary and Smith and more land in the news! Blur’s Modern Life Is Rubbish receives CMJ’s “Silver Salute.” 25 Loud Rock Reviews of Fu Manchu, Nightrage and Figure 6 Essential/Reviews Four; Iron Maiden track listing revealed; new Lifesavas, Ima Robot, Revolution Smile, Fred Ill Niño lineup announced; Zakk Wylde com- Avril, Vusi Mahlasela, Porcupine Tree, Diffuser ments on new Ozzy album; Nuclear Assault’s and Ohgr. -
Dur 24/05/2020
DOMINGO 24 DE MAYO DE 2020 2 ETCÉTERA 24/7/365 Mijares obtiene el POR: RICARDO MILLA DAMON ALBARN Y Ruiz de Alarcón COMPAÑÍA (PARTE 1 DE 2) Damon Albarn nació en 1968 y Peter Hook en 1956. El escritor go (Ujed) y está a cargo del Los separan 12 años pero duranguense fue Taller de Teatro Universita- los une su genio musical. reconocido con el rio y del Proyecto Editorial En este fatídico 2020 aca- Espacio Vacío. ban de publicar su prime- prestigiado premio de Ha realizado más de un ra colaboración ‘Aries’ fir- dramaturgia. centenar de puestas en esce- mada bajo la agrupación na presentadas dentro y fue- Gorillaz. EL SIGLO DE DURANGO ra del país a través del taller El primero debutó con Durango Espacio Vacío (que fundó en el disco titulado ‘Leisure’ 1977) y con las que consiguió en 1991 con su banda ini- Por unanimidad del jurado varios premios nacionales e cial Blur. integrado por Ximena Esca- internacionales. El cuarteto londinense lante Muñoz, Ricardo Pérez Su talento y pasión en probó las mieles del éxito Quitt y Petrona de la Cruz, el mundo de las letras han desde sus inicios y ha en- se definió entregar el Pre- dado paso a la publicación tregado discos y sencillos mio de Dramaturgia ‘Juan de más de medio centenar inolvidables, imprescindi- Ruiz de Alarcón’ 2020 en de obras de teatro (40 estre- bles. De su ópera prima se do en Reykjavík y Londres Trayectoria al poeta, narra- nadas), y numerosos libros, desprendieron “She’s So contendría su canción dor y dramaturgo duran- ensayos y prólogos sobre High” y luego con Stephen más emblemática y reco- guense Enrique Armando dramaturgia y teoría tea- Street tras los controles nocible, la breve pero in- Mijares Verdín. -

MATTHEW LONGFELLOW (K73-78) on Leaving the College He Started
MATTHEW LONGFELLOW (K73-78) On leaving the College he started work as an assistant film editor and then editor, working in the field of TV commercials and music videos. In 1989 he started directing commercials, music videos, live concerts and documentaries. He still has a love of editing and continues to edit much of his own work, as well as programmes and concerts for other directors when he’s in between projects of his own. Since 2001 he has been one of the main directors of a music documentary series that goes out on BBC2 called 'Classic Albums'. At the start of 2008 he has just finished making a programme in the forthcoming series about John Lennon's 'John Lennon Plastic Ono Band' album from 1970. He has won awards for programmes on Pink Floyd's 'Dark Side Of The Moon' and Frank Zappa's 'Overnite Sensation & Apostrophe'. He worked as both Director and Editor on the following films : Frank Zappa - Overnite Sensation & Apostrophe (2007) This focuses on Frank Zappa’s early seventies albums Over-nite Sensation (1973) and Apostrophe(’) (1974). Together they encapsulate his extraordinary musical diversity and were also the two most commercially successful albums that he released in his prolific career. The programme uses interviews, musical demonstrations, rare archive and home movie footage plus live performances to tell the story behind the conception and recording of these groundbreaking albums. Queen - 'A Night at the Opera' (2005) This documentary reveals the creative forces that lay behind Queen’s fourth album released in late 1975 and features interviews with Brian, Roger and producer Roy Thomas Baker. -

The View from Inside Hugh Hefner's Harem
14 書香人生 B O O K S & R E V I E W S SUNDAY, AUGUST 16, 2009 • TAIPEI TIMES Softcover: US CDs The view from Santigold rip-offs go, Boom, with the city’s rougher voices, like Big the debut single by Anjulie, Tuck, Tum Tum or Fat Pimp. inside Hugh As is among the cleverest. Apart Dorrough is an unmemorable rapper, from the drums, all the other instruments though; dismal rhymes abound on his debut — the flatulent junkyard brass, the bone- album, Dorrough Music. Nevertheless dry Morricone guitars — are obfuscated, he’s got a gift for the sticky concept. Hefner’s harem floating through haze. On top Anjulie coos Walk That Walk is an addictive ode to coyly about giving in when really she should catcalling. With its bouncy, high-pitched Former Bunny Girl Izabella St James paints an know better. And the faintly Caribbean synth stabs, Flashout captures the nervous unflattering picture of life at the chorus — “Boom, sha-la-ka” — echoes a energy of conspicuous consumption. And skipping heartbeat, a theme woven into the occasionally Dorrough’s overreliance on the Playboy Mansion and its lord of the manor song’s lyrics. (On Aug. 4, Boom received a simple results in cleverness. On What’s My 2009 MTV Video Music Award nomination Ringtone, he wonders what song plays when BY CAROLE CadwaLLadR for breakthrough video.) he calls his girl: “Is it Love by Keyshia Cole?/ THE OBSERVER, LONDON But unlike Santigold, who occasionally Or Playa Cardz by Keyshia Cole?/All I know forgets to ground her excursions in song is it better not be that I Should’ve Cheated Bunny Tales is subtitled “Behind craft, Anjulie isn’t eccentric in the least. -

Volume 83, No.1, January 2019 ISSN 0110-5566 (Print)
ISSN 0110-5566 (Print) ISSN 2624-1161 (Online) Volume 83, No.1, January 2019 Published on behalf of the New Zealand Institute of Chemistry in January, April, July and October. The New Zealand Institute of Chemistry Publisher Incorporated Rebecca Hurrell PO Box 13798 Email: [email protected] Johnsonville Wellington 6440 Advertising Sales Email: [email protected] Email: [email protected] Printed by Graphic Press Editor Dr Catherine Nicholson Disclaimer C/- BRANZ, Private Bag 50 908 The views and opinions expressed in Chemistry in New Porirua 5240 Zealand are those of the individual authors and are not necessarily those of the publisher, the Editorial Phone: 04 238 1329 Board or the New Zealand Institute of Chemistry. Mobile: 027 348 7528 Whilst the publisher has taken every precaution to Email: [email protected] ensure the total accuracy of material contained in Chemistry in New Zealand, no responsibility for errors or omissions will be accepted. Consulting Editor Emeritus Professor Brian Halton Copyright School of Chemical and Physical Sciences The contents of Chemistry in New Zealand are subject Victoria University of Wellington to copyright and must not be reproduced in any PO Box 600, Wellington 6140 form, wholly or in part, without the permission of the Publisher and the Editorial Board. Email: [email protected] On the cover: Mendeleev’s handwritten version of the periodic law, based on atomic weight and chemical resemblance. The United Nations have designated 2019 the International Year of the Periodic Table of Chemical Elements. It marks 150 years since Mendeleev’s first table. See article by Brian Halton, page 12. -

Britpop's Common People – National Identity, Popular Music and Young
Britpop's Common People – National identity, popular music and young people in the 1990's Claudia Lueders Doctor of Philosophy (PhD), Royal Holloway, University of London 18th November 2016 Declaration of Authorship I (Claudia Lueders) hereby declare that this thesis and the work presented in it is entirely my own. Where I have consulted the work of others, this is always clearly stated. Signed: ______________________ Date: ________________________ 2 | P a g e Britpop's Common People – National identity, popular music and young people in the 1990's Abstract: The thesis discusses the significance of Britpop’s representation of British identity for British youth and their attitude towards British identity in the 1990’s. Taking issue with the dominant academic critique of Britpop as an ‘assertion of white, male, heterosexual Englishness’ (Bennett and Stratton, 2010, p.6; Percival, 2010; Hawkins, 2010; Whiteley, 2010) the thesis argues that Britpop’s representation of national identity was more complex and ambiguous than previously suggested by academia and that Britpop’s positive attitude towards Britain and its nostalgic image of British identity needs to be interpreted as a cultural critique of social, economic and political changes in the United Kingdom in the 90s. The concepts of ‘Imagined communities’ (Anderson, 1983) and of ‘Banal nationalism’ (Billig, 1995) are used as a starting point to explore the significant role of young people and popular music for the construction and reproduction of national identity. Drawing on a qualitative textual analysis of Britpop lyrics, album reviews, and mainstream media coverage alongside data collected from qualitative interviews/surveys with musicians, PR agents, journalists, and fans, it also discusses how national identity is constructed and maintained through cultural references in popular music and related media discourses. -

Genebygeneydanabnorma
Blur Songs - Free Printable Wordsearch GENEBYGENEY DANABNORMAL BEETLEBUMUCHEMICA LWORLD SL KNOMONSTERSINME GWU OIMISSAMERICA N OER IANUL TRANOLA OEE CKNE MM DTTME ADTRACYJACKS A SHSIC EHT SHG OEO RI IEE I NDNBE THEUNIVERSALL TC GELGA OMHA CBA MBADHEADR OM AITMD EATHOFAPARTY UE RCCABES TDAYSH GR ARSON C HI EMCWLT HEMEFROMRETROTC MEOAL ERNOLDSAMEO A MLM EYOPTIGAN YF OPE CAE RC CPEACHST DTIP UA SCOOI SOLUT WR BSTON LGOLRTSN OKS IIATUGO REAISTE LRA RLRRNHA HTNOECAA D TESITKL ERHNJASPP V HNHBMNRLCU EGFIRAE BDCAAAMIYNT ROSERER AAEPTBVTAH SDIL LKT DYETDR OINHI IL DDL AUENTA BI AE BSGR UF Y BETJ E THE DEBT COLLECTOR CHARMLESS MAN TRIMM TRABB BAD HEAD HE THOUGHT OF CARS YUKO AND HIRO ERNOLD SAME CARAMEL NO MONSTERS IN ME ICE CREAM MAN SWEET SONG BAD DAY DEATH OF A PARTY BLUREMI SWAMP SONG INERTIA END OF A CENTURY THE UNIVERSAL TURN IT UP JUBILEE THEME FROM RETRO BANK HOLIDAY BEETLEBUM OPTIGAN COMMERCIAL BREAK DAN ABNORMAL GOOD SONG SILENCE CHEMICAL WORLD MISS AMERICA BEST DAYS BATTLE COME TOGETHER GENE BY GENE DANCEHALL ADVERT COUNTRY HOUSE STAR SHAPED BIRTHDAY PEACH THIS IS A LOW TRACY JACKS PARKLIFE SONG MAGIC AMERICA VILLA ROSIE ULTRANOL SING TRAILERPARK JETS Free Printable Wordsearch from LogicLovely.com. Use freely for any use, please give a link or credit if you do. Blur Songs - Free Printable Wordsearch AMBULANCE O GIRLSBOYS UCOFFEETV FORTOMORROW T H OTOTHEEND ONYOUROWN I FCL OVEROVERDOVER G TPENTER TAINMETENDER H IR NM WC ME WMO EO EBS COE RAO LSTHD LRL UUOWI FL MC SER OPNA OER TJ ELMED WDA OEYES OMASE SOZ IROBA NONEAL OWY LEU NJVB OWNNB