Greene's Tuctoria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Phylogeny of the Hubbardochloinae Including Tetrachaete (Poaceae: Chloridoideae: Cynodonteae)
Peterson, P.M., K. Romaschenko, and Y. Herrera Arrieta. 2020. A phylogeny of the Hubbardochloinae including Tetrachaete (Poaceae: Chloridoideae: Cynodonteae). Phytoneuron 2020-81: 1–13. Published 18 November 2020. ISSN 2153 733 A PHYLOGENY OF THE HUBBARDOCHLOINAE INCLUDING TETRACHAETE (CYNODONTEAE: CHLORIDOIDEAE: POACEAE) PAUL M. PETERSON AND KONSTANTIN ROMASCHENKO Department of Botany National Museum of Natural History Smithsonian Institution Washington, D.C. 20013-7012 [email protected]; [email protected] YOLANDA HERRERA ARRIETA Instituto Politécnico Nacional CIIDIR Unidad Durango-COFAA Durango, C.P. 34220, México [email protected] ABSTRACT The phylogeny of subtribe Hubbardochloinae is revisited, here with the inclusion of the monotypic genus Tetrachaete, based on a molecular DNA analysis using ndhA intron, rpl32-trnL, rps16 intron, rps16- trnK, and ITS markers. Tetrachaete elionuroides is aligned within the Hubbardochloinae and is sister to Dignathia. The biogeography of the Hubbardochloinae is discussed, its origin likely in Africa or temperate Asia. In a previous molecular DNA phylogeny (Peterson et al. 2016), the subtribe Hubbardochloinae Auquier [Bewsia Gooss., Dignathia Stapf, Gymnopogon P. Beauv., Hubbardochloa Auquier, Leptocarydion Hochst. ex Stapf, Leptothrium Kunth, and Lophacme Stapf] was found in a clade with moderate support (BS = 75, PP = 1.00) sister to the Farragininae P.M. Peterson et al. In the present study, Tetrachaete elionuroides Chiov. is included in a phylogenetic analysis (using ndhA intron, rpl32- trnL, rps16 intron, rps16-trnK, and ITS DNA markers) in order to test its relationships within the Cynodonteae with heavy sampling of species in the supersubtribe Gouiniodinae P.M. Peterson & Romasch. Chiovenda (1903) described Tetrachaete Chiov. with a with single species, T. -

Greene's Tuctoria 0 12.5 25 50 75 100
14. TUCTORIA GREENEI (GREENE’S TUCTORIA) a. Description and Taxonomy Taxonomy.—The genus Tuctoria is in the grass family (Poaceae), subfamily Chloridoideae, and is a member of the Orcuttieae tribe, which also includes Neostapfia and Orcuttia (Reeder 1965, Keeley 1998). Vasey (1891:146) originally assigned the name Orcuttia greenei to this species, from a type specimen collected in 1890 “on moist plains of the upper Sacramento, near Chico, California,” presumably in Butte County (Hoover 1941, Crampton 1958). Citing differences in lemma morphology, arrangement of the spikelets, and other differences (see “Description” below), Reeder (1982) segregated the genus Tuctoria from Orcuttia and created the new scientific name Tuctoria greenei for this species. Subsequent research suggests that Tuctoria is intermediate in evolutionary position between the primitive genus Neostapfia and the advanced genus Orcuttia (Keeley 1998, L. Boykin in litt. 2000). Several other common names have been used for this species, including Chico grass (Scribner 1899), awnless Orcutt grass (Abrams 1940), Greene’s orcuttia (Smith et al. 1980), and Greene’s Orcutt grass (California Department of Fish and Game 1991, U.S. Fish and Wildlife Service 1985c). Description and Identification.—The basic characteristics pertaining to all members of the Orcuttieae were described above in the Neostapfia colusana account. The genus Tuctoria is characterized by flattened spikelets similar to those of Orcuttia species, except that the spikelets of Tuctoria grow in a spiral, as opposed to a distichous, arrangement. Tuctoria species have short-toothed, narrow lemmas. The juvenile and terrestrial leaves of Tuctoria are similar to those of Orcuttia, but Tuctoria does not produce the floating type of intermediate leaves (Reeder 1982, Keeley 1998). -

Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(S): Grass Phylogeny Working Group, Nigel P
Phylogeny and Subfamilial Classification of the Grasses (Poaceae) Author(s): Grass Phylogeny Working Group, Nigel P. Barker, Lynn G. Clark, Jerrold I. Davis, Melvin R. Duvall, Gerald F. Guala, Catherine Hsiao, Elizabeth A. Kellogg, H. Peter Linder Source: Annals of the Missouri Botanical Garden, Vol. 88, No. 3 (Summer, 2001), pp. 373-457 Published by: Missouri Botanical Garden Press Stable URL: http://www.jstor.org/stable/3298585 Accessed: 06/10/2008 11:05 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=mobot. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit organization founded in 1995 to build trusted digital archives for scholarship. We work with the scholarly community to preserve their work and the materials they rely upon, and to build a common research platform that promotes the discovery and use of these resources. For more information about JSTOR, please contact [email protected]. -

Biological Resources Assessment the Ranch ±530- Acre Study Area City of Rancho Cordova, California
Biological Resources Assessment The Ranch ±530- Acre Study Area City of Rancho Cordova, California Prepared for: K. Hovnanian Homes October 13, 2017 Prepared by: © 2017 TABLE OF CONTENTS 1.0 Introduction ......................................................................................................................... 1 1.1. Project Description ........................................................................................................... 1 2.0 Regulatory Framework ........................................................................................................ 2 2.1. Federal Regulations .......................................................................................................... 2 2.1.1. Federal Endangered Species Act ............................................................................... 2 2.1.2. Migratory Bird Treaty Act ......................................................................................... 2 2.1.3. The Bald and Golden Eagle Protection Act ............................................................... 2 2.2. State Jurisdiction .............................................................................................................. 3 2.2.1. California Endangered Species Act ........................................................................... 3 2.2.2. California Department of Fish and Game Codes ...................................................... 3 2.2.3. Native Plant Protection Act ..................................................................................... -

US EPA-Pesticides; Dodine
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON D.C., 20460 OFFICE OF PREVENTION, PESTICIDESDES AND TOXIC SUBSTANCES PC Code: 044301 DP Barcode: D338148 Date: January 22, 2008 MEMORANDUM SUBJECT: Ecological Risk Assessment for the Dodine Section 3 New Use on Peanuts and Bananas TO: Robert Westin, Product Manager Mary Waller, Team Leader Registration Division (7505P) FROM: Christopher J. Salice, P.h.D, Biologist Marietta Echeverria, Envronmental Scientist Environmental Risk Branch IV Environmental Fate and Effects Division (7507P) REVIEWED BY: Thomas Steeger, Ph.D., Senior Biologist R. David Jones, Ph.D., Senior Agronomist Environmental Risk Branch IV Environmental Fate and Effects Division (7507P) APPROVED BY: Elizabeth Behl, Branch Chief Environmental Risk Branch IV Environmental Fate and Effects Division (7507P) The Environmental Fate and Effects Division (EFED) has reviewed the proposed label for the use of dodine (n-dodecylguanidine monoacetate; CAS 2439-10-3) and its end-use product SYLLIT® FL (39.6% dodine) fungicide on peanuts and bananas. The results of this screening-level risk assessment indicate that the proposed new uses of dodine on peanuts and bananas have the potential for direct adverse effects on listed and non-listed freshwater and estuarine/marine invertebrates, listed and non-listed vascular and non-vascular plants, and listed and non-listed birds and mammals. Major data gaps are listed below. Without these data potential risk to the associated taxa can not be precluded: • Aquatic vascular plant toxicity data (850.4400) There is uncertainty regarding the potential chronic effects of dodine to saltwater invertebrates and fish since there are no toxicity data. Using acute-to-chronic ratios (ACR) from freshwater species to calculate chronic endpoints for the saltwater species, however, suggests that risks may be low. -

Polypogon Monspeliensis (L.) Desf., 1798, CONABIO, Junio 2016 Polypogon Monspeliensis (L.) Desf., 1798
Método de Evaluación Rápida de Invasividad (MERI) para especies exóticas en México Polypogon monspeliensis (L.) Desf., 1798, CONABIO, Junio 2016 Polypogon monspeliensis (L.) Desf., 1798 Foto: Philipp Weigell, 2013. Fuente: Wikimedia Polypogon monspeliensis es una hierba anual reportada como invasora en varios países (PIER, 2011), al parecer esta especie es huésped del nematodo Anguina sp . el cual es vector de la bacteria Clavibacter toxicus , productor de la corynetoxina causante de la muerte del ganado conocida como toxicidad de ballica anual (ARGT) (Halvorson & Guertin, 2003; McKay et al., 1993), en Estados Unidos P. monspeliensis ha afectado a Orcuttia inaequidens, Orcurttia pilosa y Tuctoria greenei , especies con categoría de riesgo (CABI, 2014). Información taxonómica Reino: Plantae División: Tracheophyta Clase: Magnoliopsida Orden: Poales Familia: Poaceae Género: Polypogon Especie: Polypogon monspeliensis (L.) Desf., 1798 Nombre común: rabo de cordero, rabo de zorra, cola de zorro (Secretaria Distrital de Ambiente, 2009). 1 Método de Evaluación Rápida de Invasividad (MERI) para especies exóticas en México Polypogon monspeliensis (L.) Desf., 1798, CONABIO, Junio 2016 Valor de invasividad: 0.4656 Categoría de riesgo : Alto Descripción de la especie Polypogon monspeliensis es una hierba anual con tallos y hojas envainantes y alternas, lígula membranosa. La Inflorescencia en panícula densa, oblongoidea, sedosa, a veces lobada. Las espiguillas con una flor hermafrodita, pedúnculos articulados en la parte superior, 2 glumas subiguales, mayores que las flores, emarginadas, aristadas, con espículos cónicos en la base. Lemas dentadas, con arista terminal. Con tres estambres (Secretaria Distrital de Ambiente, 2009). se reproduce por semillas que son dispersadas por animales (CABI, 2014; PIER, 2011). Distribución original Originario de Europa, África y Asia. -

Viruses Virus Diseases Poaceae(Gramineae)
Viruses and virus diseases of Poaceae (Gramineae) Viruses The Poaceae are one of the most important plant families in terms of the number of species, worldwide distribution, ecosystems and as ingredients of human and animal food. It is not surprising that they support many parasites including and more than 100 severely pathogenic virus species, of which new ones are being virus diseases regularly described. This book results from the contributions of 150 well-known specialists and presents of for the first time an in-depth look at all the viruses (including the retrotransposons) Poaceae(Gramineae) infesting one plant family. Ta xonomic and agronomic descriptions of the Poaceae are presented, followed by data on molecular and biological characteristics of the viruses and descriptions up to species level. Virus diseases of field grasses (barley, maize, rice, rye, sorghum, sugarcane, triticale and wheats), forage, ornamental, aromatic, wild and lawn Gramineae are largely described and illustrated (32 colour plates). A detailed index Sciences de la vie e) of viruses and taxonomic lists will help readers in their search for information. Foreworded by Marc Van Regenmortel, this book is essential for anyone with an interest in plant pathology especially plant virology, entomology, breeding minea and forecasting. Agronomists will also find this book invaluable. ra The book was coordinated by Hervé Lapierre, previously a researcher at the Institut H. Lapierre, P.-A. Signoret, editors National de la Recherche Agronomique (Versailles-France) and Pierre A. Signoret emeritus eae (G professor and formerly head of the plant pathology department at Ecole Nationale Supérieure ac Agronomique (Montpellier-France). Both have worked from the late 1960’s on virus diseases Po of Poaceae . -

Report of Science Advisors
Report of Science Advisors Solano County Natural Community Conservation Plan Habitat Conservation Plan November 2002 Reed Noss (Lead Reviewer and Editor), Ronald Amundson, Dick Arnold, Michael Bradbury, Sharon Collinge, Brenda Grewell, Richard Grosberg, Lester McKee, Phil Northen, Christina Swanson, and Ron Yoshiyama Facilitators: Bruce DiGennaro and Vance Russell TABLE OF CONTENTS Executive Summary.........................................................................................................................1 1.0 Introduction...............................................................................................................................5 1.1 Role of Science Advisors..............................................................................................5 1.2 Science Advisors Workshop.........................................................................................6 1.3 Report Organization......................................................................................................7 2.0 Regional and Historical Context...............................................................................................7 2.1 Biodiversity of the Region............................................................................................8 2.2 Geography and Geology ...............................................................................................9 2.3 Climate and Hydrology...............................................................................................14 3.0 Data Gaps and -

Vascular Plants of Vina Plains Preserve Wurlitzer Unit
DO r:r:i :;:i·,iOVE Ff.'f)l,1 . ,- . - . "'I":; Vascular Plants of Vina Plains Preserve, Wurlitzer Unit Vernon H. Oswald Vaseular Plants of Vina Plains Preserve, Wurlitzer Unit Vernon H . Oswald Department of Biological Sciences California State University, C h ico Ch ico, California 95929-0515 1997 Revision RED BLUFF •CORN ING TEHAMA CO. -------------B1JITECO. ORLAND HWY 32 FIGURE I. Location of Vina P la.ins Preserve, Main Unit on the north, Wurlitzer Unit on the south. CONTENTS Figure 1. Location of Vina Plains Preserve ...... ................................. facing contents Figure 2. Wurlitzer Unit, Vina Plains Preserve ..... ............................... facing page I Introduction .. ... ..................................... ................................ ....... ....... ... ................ I References .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. ... ... .. .. .. .. .. .. .. .. .. .. 4 The Plant List: Ferns and fem allies .......... ................................................... ........... ................. 5 Di cot flowering plants ............... ...................................................... ................. 5 Monocot flowering plants .......... ..................................................................... 25 / ... n\ a: t, i. FIGURE 2. Wurlitzer Unit, Vina Plains Preserve (in yellow), with a small comer of the Main Unit showing on the north. Modified from USGS 7.5' topographic maps, Richardson Springs NW & Nord quadrangles. - - INTRODUCTION 1 A survey of the vascular flora of the Wurl itzer -

4.4 BIOLOGICAL RESOURCES 4.4.1 Regulatory Setting
Ascent Environmental Administrative Draft – For Internal Review and Deliberation Biological Resources 4.4 BIOLOGICAL RESOURCES This section describes the potential effects of the project on biological resources. This section also addresses biological resources known or with potential to occur in the project vicinity, including common vegetation and habitat types, sensitive plant communities, and special-status plant and animal species. The analysis includes a description of the existing environmental conditions, the methods used for assessment, the potential direct and indirect impacts of project implementation not included in the 2005 Subsequent Environmental Impact Report (SEIR) for the Hay Road Landfill Project (Solano County 2005), and mitigation measures recommended to address impacts determined to be significant or potentially significant. The data and documents reviewed in preparation of this analysis included: 2005 SEIR for the Hay Road Landfill Project (Solano County 2005); Burrowing Owl Habitat Assessment (ESA 2016a); California Tiger Salamander Habitat Assessment (ESA 2016b); Branchiopod Survey Report (ESA 2016c); Special-status Plant Survey Report (ESA 2016d); Organics Transload Facility Habitat Assessment (ESA 2017a); Hydro Flow Analysis (ESA 2017b); Contra Costa Goldfields Survey Report (ESA 2017c); Delta Green Ground Beetle Survey Report and Supplemental Habitat Assessment Report (Entomological Consulting Services, Ltd. 2016, 2018); reconnaissance-level survey of the project site conducted on August 7, 2017; records search and GIS query of the California Natural Diversity Database (CNDDB) within 5 miles of the project site (2018); California Native Plant Society (CNPS), Rare Plant Program database search of the Allendale, Dixon, Saxon, Elmira, Dozier, Liberty Island, Denverton, Birds Landing, and Rio Vista U.S. Geological Service 7.5-minute quadrangles (CNPS 2018); eBird online database of bird observations (eBird 2018); and aerial photographs of the project site and surrounding area. -

Cramvernal Pool Endemics-Final.Pdf
Vernal Pool Systems and Individual Vernal Pools Version 6.1 APPENDIX 1 Vernal Pool Endemic Plant List Use this list to determine if a species is a vernal pool endemic Bsed on Appendix C from: T. Keeler-Wolf, D.R. Elam, K. Lewis, S.A. Flint. 1998. California Vernal Pool Assessment Preliminary Report. State of California, The Resources Agency, Department of Fish and Game. 161 pp. www.dfg.ca.gov/biogeodata/wetlands/pdfs/VernalPoolAssessmentPreliminaryReport.pdf May 2013 ! CRAM%Vernal%Pool%Endemic%Plants%List May%2013 Scientific%Name Family Genus Species infraspecific_rank %infraspecific_epithet Agrostis(elliottiana POACEAE Agrostis elliottiana Agrostis(hendersonii POACEAE Agrostis hendersonii Agrostis(microphylla POACEAE Agrostis microphylla Alopecurus(carolinianus POACEAE Alopecurus carolinianus Alopecurus(saccatus POACEAE Alopecurus saccatus Anagallis(minima MYRSINACEAE Anagallis minima Astragalus(tener(var.(ferrisiae FABACEAE Astragalus tener var. ferrisiae Astragalus(tener(var.(tener FABACEAE Astragalus tener var. tener Atriplex(cordulata CHENOPODIACEAE Atriplex cordulata Atriplex(cordulata(var.(cordulata CHENOPODIACEAE Atriplex cordulata var. cordulata Atriplex(cordulata(var.(erecticaulis CHENOPODIACEAE Atriplex cordulata var. erecticaulis Atriplex(depressa CHENOPODIACEAE Atriplex depressa Atriplex(minuscula CHENOPODIACEAE Atriplex minuscula Atriplex(parishii CHENOPODIACEAE Atriplex parishii Atriplex(persistens CHENOPODIACEAE Atriplex persistens Atriplex(subtilis CHENOPODIACEAE Atriplex subtilis Blennosperma(bakeri ASTERACEAE Blennosperma -
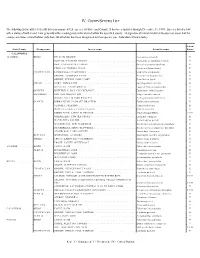
Iv. County/Species List
IV. COUNTY/SPECIES LIST The following list identifies federally listed or proposed U.S. species by State and County. It has been updated through December 31, 1999. Species listed below with a status of both E and T are generally either endangered or threatened within the specified county. Designation of critical habitat (CH) does not mean that the county constitutes critical habitat, only that critical habitat has been designated for that species (see Addendum A Instructions). Action/ State/County Group name Inverse name Scientific name Status CALIFORNIA ALAMEDA ......... BIRDS ....... PELICAN, BROWN ........................................ Pelicanus occidentalis E PLOVER, WESTERN SNOWY ................................ Charadrius alexandrinus nivosus T RAIL, CALIFORNIA CLAPPER ............................... Rallus longirostris obsoletus E TERN, CALIFORNIA LEAST ................................. Sterna antillarum browni E CRUSTACEAN . LINDERIELLA, CALIFORNIA ................................ Linderiella occidentalis E SHRIMP, LONGHORN FAIRY ................................ Branchinecta longiantenna E SHRIMP, VERNAL POOL FAIRY .............................. Branchinecta lynchi T FISHES ...... GOBY, TIDEWATER ...................................... Eucyclogobius newberryi E SPLITTAIL, SACRAMENTO ................................. Pogonichthys macrolepidotus T INSECTS ..... BUTTERFLY, BAY CHECKERSPOT ........................... Euphydryas editha bayensis T MAMMALS ... FOX, SAN JOAQUIN KIT .................................... Vulpes macrotis