Andhra Medi Pharma India Pvt. Ltd. Sy.No
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of SSP.Pdf
List of Self-Sealing Permission granted by the Commissioner of Customs (Preventive), Vijayawada [ From 01.01.2018 to 31.07.2018] Sl.N Names of Authorised Sigantories for SSP No. Date of Issue Name of Applicant Address of Applicant IEC GSTIN Address of Sealing Premises o. sealing allotted of SSP 1 2 3 4 5 6 7 8 9 1 Siri Smelters & Energy plot no. 262/B & 263/A, APIIC Growth Centre, Bobbili (i) Sajja Jyothsna Sree (ii) Sajja Venkateswara plot no. 262/B & 263/A, APIIC Growth 0912015829 37AAPCS2941A1ZE 90/2017 02.01.2018 Private Limited -535558 Rao Centre, Bobbili -535558 2 PLR Foods Private Sy. No. 354/1E, Ranganatha Mitta Village, Sodum 0911001352 37AAFCP9113P1ZU [1] P. Sudhir Reddy [2] P Indira Reddy Sy. No. 354/1E, Ranganatha Mitta Village, 02.01.2018 Limited Mandal & Post, Chittoor District - 517123, AP Sodum Mandal & Post, Chittoor District - 01/2018 517123, AP 3 Kalyan Aqua & Marine SY. No. 143, 144/1 to 3, Keerthipadu Village, 2604000857 37AADCK2221M1ZK [1] Putchakayala Seshadri Choudhary, SY. No. 143, 144/1 to 3, Keerthipadu 02.01.2018 Exports India (P) Ltd Maddipadu Mandal, Prakasam District - 523211, AP Director Village, Maddipadu Mandal, Prakasam 02.2018 District - 523211, AP 4 Chakri Fisheries Private SY. No. 143, 144/1 to 3, Keerthipadu Village, 2614000230 37AAFCC6232L1ZH [1] Putchakayala Sireesha, Director SY. No. 143, 144/1 to 3, Keerthipadu 02.01.2018 Limited Maddipadu Mandal, Prakasam District - 523211, AP Village, Maddipadu Mandal, Prakasam 03/2018 District - 523211, AP 5 Hind Granite Pvt. Ltd, Sy. No. 1024, 1028/4, Chimakurthy Village & Mandal, 0916502091 37AADCH8796E1Z4 [1] D. -

2019071371.Pdf
.:€ ' Proceedings of the Collector & District Magistrate. Krishna, Machilipatnam Present: Sri A.MD. lMTlAZ, 1.A.5.. >kJ.* REV-A5ECoPT(VRO)/3 /2o1s-sA-(A7)-KCo Dated: l0 .07.2019. Sub: Village Revenue Officers - Transfers and Postings of Employees - Revenue Department - Krishna District - Orders - lssued. Read:- 6.O.Ms, No. 45 Finance (HR l-P16. & POLICY) Department, Dt.:24.06.2019. ,( :k )k ORDER: {n the reference read above, the Government have relaxed ban orders imposed on transfer of the employees. ln pursuance to the orders of the Government issued in the reference read above, the following Village Revenue Officers are hereby transferred from their working places and posted to the places in Vijayawada Division as shown against them: :' Name of the VRO & present Place of Transferred and posted as 5l.No. Division Working VRO, K.Butchaiah, Kanuru, O/o Tahsildar, Dabbakupalli (V), Vatsavai I VIJAYAWADA Machilipatnam Mandal K Praveen, VRO, Purushothampatnam, 6arlnavaram (M),O/o Tahsildar Ketanakonda (V), lbrahimpatnam 2 VIJAYAWADA Gannavaram,VRO, Purushothampatnam, (M) Gannavaram (M) P Mary Latha, VRO, Telaprolu, Unguturu 3 (M),O/o Tahsildar Unguturu,VRO, Uppuluru-2 (V), Kankipadu (M) VIJAYAWADA Telaprolu, Unguturu (M) GURVINDAPALLI MOHAN RAO, VRO, 6andigunta-1, Vuyyuru Mandal,O/o 4 Vanukuru (V), Penamaluru (M) VIJAYAWADA TaLxildar Vuyyuru, VRO, Gandigunta-1, Vuwuru Mandal RAYALA RAMADEVI, VRO, Chinaogirala (V), Vuyyuru (M),O/o Tahsildar Punadipadu-ll Village, Kankipadu 5 VIJAYAWADA Vuyyuru,VRO, Chinaogirala (V), Vuyyuru Mandal (M) P-PAVAN KUMAR, VRO, Gopavaram-|, Enikepadu Village of Vijayawada 6 Musunuru,O/o Tahsildar Musunuru,VRO, VIJAYAWADA Rural Gopavaram-|, Musunuru VRO, Vavi lala (V), R.Venkateswararao, Kondapallivillage of 7 Tiruvuru,O/o Tahsildar Tiruvuru, VRO, VIJAYAWADA lbrahimpatnam Mandal Vavilala(V), Tiruvuru M.fhantibabu, VRO, Pamidimukkala,O/o Northvalluru I of Thotlavalluru 8 Tahsildar Pamidimukkala.VRO. -

District Survey Report - 2018
District Survey Report - 2018 4 DEPARTMENT OF MINES AND GEOLOGY Government of Andhra Pradesh DISTRICT SURVEY REPORT - KRISHNA DISTRICT Prepared by ANDHRA PRADESH SPACE APPLICATIONS CENTRE (APSAC) ITE & C Department, Govt. of Andhra Pradesh 2018 i District Survey Report - 2018 ACKNOWLEDGEMENTS APSAC wishes to place on record its sincere thanks to Sri. B.Sreedhar IAS, Secretary to Government (Mines) and the Director, Department of Mines and Geology, Govt. of Andhra Pradesh for entrusting the work for preparation of District Survey Reports of Andhra Pradesh. The team gratefully acknowledge the help of the Commissioner, Horticulture Department, Govt. of Andhra Pradesh and the Director, Directorate of Economics and Statistics, Planning Department, Govt. of Andhra Pradesh for providing valuable statistical data and literature. The project team is also thankful to all the Joint Directors, Deputy Directors, Assistant Directors and the staff of Mines and Geology Department for their overall support and guidance during the execution of this work. Also sincere thanks are due to the scientific staff of APSAC who has generated all the thematic maps. VICE CHAIRMAN APSAC ii District Survey Report - 2018 Contents Page Acknowledgements List of Figures List of Tables 1 Salient Features of Krishna District 1 1.1 Administrative Setup 1 1.2 Drainage 2 1.2a Kolleru Lake- A eco-sensitive zone 4 1.3 Climate and Rainfall 4 1.4 Transport and Communications 9 1.5 Population and Literacy 10 1.6 Important Places 11 1.6a Places of Tourist Interest 11 1.6b Places of -

Krishna District Machilipatnam Ph-223602 Krishna District Cell-9885395597 12
11. SRI KOMPELLA GOPAL MACHILIPATNAM RICE MILL,SARPANCH VARI STREET BUTTAI PETA 1. SRI KALAPU GOPALAKRISHNA MURTHY MACHILIPATNAM D NO-5/323-1 ,JAVVARUPET KRISHNA DISTRICT MACHILIPATNAM PH-223602 KRISHNA DISTRICT CELL-9885395597 12. SRI GHANTASALA VENKATA SUBBA RAO PH - 08672 227602 , 220663 PANJA SIDE STREET,KENNADI ROAD RAMANAIDU PET 2. SRI KALAPU LAKSHMI SATYANARAYANA MACHILIPATNAM CIRCLE PETA , MACHILIPATNAM KRISHNA DISTRICT KRISHNA DISTRICT PH-229600 CELL-9440317352 , 08672 225478 13. SRI GAMINI SRINIVAS 3. SRI KALAPU GANESH KOTESWARARAO PANJA SIDE STREET,KENNADI ROAD D NO-5/323-1 ,JAVVARUPET RAMANAIDU PET MACHILIPATNAM MACHILIPATNAM KRISHNA DISTRICT KRISHNA DISTRICT PH, 08672 227602 PH-229600 4. SRI KALAPU PRASAD RAO 14. SRI VEERAMALLU GOPAL MANGALI VAARI STREET ENGLISH PALEM CENTRE CIRCLE PETA , MACHILIPATNAM MACHILIPATNAM KRISHNA DISTRICT KRISHNA DISTRICT CELL- 9440107161 PH-226852 5. SRI KALAPU DURGA NAGA 15. SRI VEERAMALLU SRINIVASARAO MALLESWARARAO D NO-22/85-1 ,BACHU PET MANGALI VAARI STREET MACHILIPATNAM CIRCLE PETA , MACHILIPATNAM KRISHNA DISTRICT KRISHNA DISTRICT CELL-9948264551 6. SRI KALAPU PURNACHANDRARAO 16. SRI KUKUNURU GOPAL (BABURAO) D NO-22-85 , ,BACHU PET OPP TO;-NARASIMHASWAMY TEMPLE MACHILIPATNAM, KRISHNA DISTRICT JALAL PET ;,MACHILIPATNAM KRISHNA DISTRICT 17. SRI YADAVALLY MURALI CELL-9290625731 D NO -7 /221 – 3 ,PADMAPRIYA BULDINGS KANYAKA RICE MILL ROAD, GODUGUPET, 7. SRI KALAPU SANKARA SOMAIAH MACHILIPATNAM KENNADI ROAD,JAVVARUPET KRISHNA DISTRICT MACHILIPATNAM CELL-9848263624 KRISHNA DISTRICT CELL-9966632242 18. SRI PENDYALA GANESH BABU PARASUPETA 8. SRI VEERAMALLU NAGAKRISHNATEJA MACHILIPATNAM KRISHNA DISTRICT 9. SRI DEVAGANUGALA VENKATA SATYA PH-251763 SUBRAMANYAM RICE MILL,SARPANCH VARI STREET 19. SRI GHANTASALA NAGARAJU BUTTAI PETA, MACHILIPATNAM PLAT NO 17 ,NGO’S COLONY KRISHNA DISTRICT PARASUPETA, CHILAKALAPUDI PH-223602 MACHILIPATNAM KRISHNA DISTRICT 10. -

Assessment of Ground Water Quality Using Water Quality Index
International Journal of Innovative Research in Advanced Engineering (IJIRAE) ISSN: 2349-2163 Issue 3, Volume 2 (March 2015) www.ijirae.com ASSESSMENT OF GROUND WATER QUALITY USING WATER QUALITY INDEX K. Sundara Kumar1*, Ch. Satish Kumar2, K. Hari Prrasad3, B. Rajesh4, R. Sivaram Prasad5 , T. Venkatesh6 1Associate Professor and Head, Department of Civil Engineering,2-6 Final B.Tech., Civil, Usha Rama College of Engineering & Technology, Telaprolu, Krishna District, Andhra Pradesh, India. ABSTRACT-- Ground water is one of the important fresh water resources available for human consumption. Early people recognized the importance of water from a quantity view point. However assessment of the quality of ground water is essential for improving its potability. In this project work we have estimated the water quality of Bapulapadu Mandal which is located 10km away from the Gannavaram airport on east coast of Krishna district of Andhra Pradesh, India. Water quality is tested for about 10 physico-chemical parameters of 90 water samples collected from various locations of 27 villages from Bapulapadu Mandal. The ground water samples are collected manually from the bore wells which were approximately equally distributed all over 27 villages of Bapulapadu Mandal. The samples were analyzed using MULTI- PARAMETER TEST KIT, which provide quick and easy water quality testing in the field. The combined effect of all there parameters was expressed in terms of WATER QUALITY INDEX. Bapulapadu Mandal map has been collected and analyzed. The data base obtained from water quality testing is used as attribute data base for preparation of thematic maps showing the spatial distribution of various water quality parameters. -

Chapter Iv Land Revenue
CHAPTER IV LAND REVENUE 4.1 Results of Audit Test check of records of Land revenue offices conducted during the year 2004-05 revealed non/short levy of revenue amounting to Rs.41.10 crore in 304 cases which broadly fall under the following categories: (Rupees in crore) Sl. Nature of irregularity No. of Amount No. cases 1 Non/short levy of water rate/land revenue 56 5.90 2 Non/short of non agricultural land assessment 11 0.35 3 Non/short levy of road cess 38 0.60 4 Non adjustment of local cess 1 0.40 5 Non recovery of regularisation charges for 4 0.09 encroachment 6 Alienation of Government lands, non recovery of 6 1.05 market value 7 Recovery of dues under Revenue Recovery Act 13 22.69 8 Non levy of interest on arrears of land revenue 138 9.21 9 Other irregularities 37 0.81 TOTAL 304 41.10 During the year 2004-05, the Department accepted under assessments etc., of Rs.1.25 lakh in three cases pertaining to earlier years. The same was realised during the year. A few illustrative cases involving Rs.3.93 crore are mentioned in the following paragraphs: Audit Report (Revenue Receipts) for the year ended 31 March 2005 4.2 Short levy of water tax As per the provisions of AP Water Tax Act, 1988, water tax is leviable for each fasli# year on all lands receiving water from any Government notified source. For this purpose, all major and medium irrigation projects are to be regarded as category-I while other Government sources which supply water for not less than four months in a year are regarded as category-II. -

Meos & MIS Co-Ordinators
List of MEOs, MIS Co-orfinators of MRC Centers in AP Sl no District Mandal Name Designation Mobile No Email ID Remarks 1 2 3 4 5 6 7 8 1 Adilabad Adilabad Jayasheela MEO 7382621422 [email protected] 2 Adilabad Adilabad D.Manjula MIS Co-Ordinator 9492609240 [email protected] 3 Adilabad ASIFABAD V.Laxmaiah MEO 9440992903 [email protected] 4 Adilabad ASIFABAD G.Santosh Kumar MIS Co-Ordinator 9866400525 [email protected] [email protected] 5 Adilabad Bazarhathnoor M.Prahlad MEO(FAC) 9440010906 n 6 Adilabad Bazarhathnoor C.Sharath MISCo-Ord 9640283334 7 Adilabad BEJJUR D.SOMIAH MEO FAC 9440036215 [email protected] MIS CO- 8 Adilabad BEJJUR CH.SUMALATHA 9440718097 [email protected] ORDINATOR 9 Adilabad Bellampally D.Sridhar Swamy M.E.O 7386461279 [email protected] 10 Adilabad Bellampally L.Srinivas MIS CO Ordinator 9441426311 [email protected] 11 Adilabad Bhainsa J.Dayanand MEO 7382621360 [email protected] 12 Adilabad Bhainsa Hari Prasad.Agolam MIS Co-ordinator 9703648880 [email protected] 13 Adilabad Bheemini K.Ganga Singh M.E.O 9440038948 [email protected] 14 Adilabad Bheemini P.Sridar M.I.S 9949294049 [email protected] 15 Adilabad Boath A.Bhumareedy M.E.O 9493340234 [email protected] 16 Adilabad Boath M.Prasad MIS CO Ordinator 7382305575 17 Adilabad CHENNUR C.MALLA REDDY MEO 7382621363 [email protected] MIS- 18 Adilabad CHENNUR CH.LAVANYA 9652666194 [email protected] COORDINATOR 19 Adilabad Dahegoan Venkata Swamy MEO 7382621364 [email protected] 20 -

Hand Book of Statistics 2018 Krishna District
HAND BOOK OF STATISTICS 2018 KRISHNA DISTRICT Compiled by CHIeF PlANNINg OFFICeR KRISHNA, MACHIlIPATNAM Sri B.LAKSHMIKANTHAM, I.A.S., Collector & District Magistrate Krishna District P R E F A C E I am glad that the Hand Book of Statistics 2018 of Krishna District with statistical dataof various departments for the year 2017-18 is being released. The statistical data in respect of various schemes being implemented by the departmentsin the district are compiled in a systematic manner so as to reflect the progress made under various sectors during the year. The sector wise progress is depicted in sector – wise tables apart from Mandal - wise data. I am confident that the publication will be of immense utility as a reference book to general public and Government and Non-Governmental agencies in general as well as Administrators, Planners, Research Scholars, Funding agencies, Banks and Non-Profit Institutions. I am thankful to all the District Officers and the Heads of Institutions for extendingtheirco-operation by furnishing the information to this Hand Book. I appreciate the efforts made by Sri T.Hima Prabhakar Raju, Chief Planning Officer (FAC), Krishna District and their Staff in collection and compilation of data in bringing out this publication. Any suggestions aimed at improvement of Hand Book are most welcome and maybe sent to the Chief Planning Officer, Krishna District at Machilipatnam Date: Station: Machilipatnam OFFICERS AND STAFF ASSOCIATED WITH THE PUBLICATION 1. SRI T.HIMA PRABHAKARA RAJU : CHIEF PLANNINg OFFICER 2. SRI M.SATYANARAYANA : STATISTICAL OFFICER 3. SRI M.ANAND KUMAR : DEPUTY STATISTICAL OFFICER, COMPILED AND COMPUTERIzED * * * DISTRICT PROFILE :: KRISHNA DISTRICT GENERAL AND PHYSICAL FEATURES Krishna District with its district head quarters at Machilipatnam is the coastal district of Andhra Pradesh. -
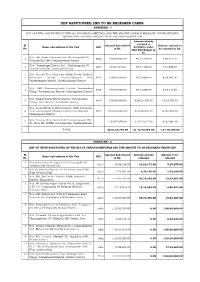
Iidf Sanctioned and to Be Released Cases Annexure - I
IIDF SANCTIONED AND TO BE RELEASED CASES ANNEXURE - I LIST OF UNITS SANCTIONED BY THE SLC IN VARIOUS MEETINGS AND THE AMOUNT ALREADY RELEASED UNDER HUD HUD BUDGET AND BALANCE AMOUNT TO BE RELEASED UNDER IIDF Amount already released @ Sl. Amount Sanctioned Balance amount to Name and address of the Unit SLC 93.2366% under No. in Rs. be released in Rs. Hud Hud Budget in Rs. M/s. My Home Industries Ltd., Mulakalapally (V), 1 86th 1,00,00,000.00 93,23,288.00 6,76,712.00 Yelamanchili (M), Visakhapatnam district M/s. Vijayanagar Biotech Ltd., Kothakopperla (V), 2 86th 78,65,115.00 73,33,166.00 5,31,949.00 Poosapatirega (M), Vizianagaram Dist. M/s. Deccan Fine Chemicals (India) Private Limited, 3 Kesavaram Village, Venkatanagaram Post, 94th 1,00,00,000.00 93,23,660.00 6,76,340.00 Payakaraopeta Mandal, Visakhapatnam District M/s. SMS Pharmaceuticals Limited, Kandivalasa 4 94th 1,00,00,000.00 93,23,660.00 6,76,340.00 Village, Poosapatirega Mandal, Vizianagaram District M/s. Trimex Sands Private Limited, Vatchavalasa 5 98th 5,00,00,000.00 4,66,18,300.00 33,81,700.00 Village, Gara Mandal, Srikakulam District M/s. Sarda Metals & Alloys Limited, APIIC Industrial 6 Park, Kantakapalli Village & Kothavalasa Mandal, 98th 10,00,00,000.00 9,32,36,600.00 67,63,400.00 Vizianagaram District M/s. Toyotsu Rare Earths India Private Limited, Plot 7 4,52,97,678.00 4,22,34,015.00 30,63,663.00 No. -

Andhra Pradesh Capital Region Development Authority Act, 2014 - Declaration of A.P
GOVERNMENT OF ANDHRA PRADESH ABSTRACT Municipal Administration & Urban Development Department - Andhra Pradesh Capital Region Development Authority Act, 2014 - Declaration of A.P. Capital Region – Orders - Issued. MUNICIPAL ADMINISTRATION & URBAN DEVELOPMENT (M2) DEPARTMENT G.O.MS.No. 253 Dated: 30.12.2014 Read the following: 1. Andhra Pradesh Capital Region Development Authority Act, 2014 (Act.No.11 of 2014 ) 2. G.O.Ms.No.252, MA&UD Dept., Dated:30.12.2014 ***** ORDER: The Andhra Pradesh Capital Region Development Authority Act, 2014 has come into force with effect from 30th day of December, 2014 by virtue of notification published in the Extra-ordinary issue Andhra Pradesh Gazette, dated: 30.12.2014. 2. The Government have consulted experts in urban development and various public organizations with regard to appropriate location of capital city. The Government have also considered various aspects of location of the capital keeping in view welfare of the state population, administrative efficiency and accessibility to all corners of the state. 3. The Government in exercise of the powers under sub section 1 of Section 3 of Andhra Pradesh Capital Region Development Authority Act, 2014 hereby notify the areas covering broadly an area of about 7068 Sq. Kms as detailed in the schedule to the notification appended here to, as Andhra Pradesh Capital Region which is meant for development under the provisions of the Andhra Pradesh Capital Region Development Authority Act-2014. 4. The appended notification shall be published in the Extra-ordinary issue of Andhra Pradesh Gazette dated: 30.12.2014. The Commissioner, Printing, Stationery & Stores Purchase, Hyderabad is requested to arrange to publish the said notification accordingly and furnish 150 copies of the notification to the Government. -

Krishna District Annual Report
Krishna District Annual Report 1st April 2018 to 31st March 2019 CONTENTS FOREWORD FROM THE DISTRICT PRESIDENT SRI SATHYA SAI SEVA ORGANISATIONS – AN INTRODUCTION WINGS OF THE ORGANISATIONS ADMINISTRATION OF THE ORGANISATION THE 9 POINT CODE OF CONDUCT AND 10 PRINCIPLES SRI SATHYA SAI SEVA ORGANISATIONS, [KRISHNA DISTRICT] BRIEF HISTORY DIVINE VISIT OVERVIEW SAI CENTRES ACTIVITIES OFFICE BEARERS SPECIFIC SERVICE PROJECTS OR INITIATIVES IMPORTANT EVENTS OR CONFERENCES GLIMPSES OF ACTIVITIES This report is dedicated at the Lotus Feet of our Lord and Master Bhagavan Sri Sathya Sai Baba Foreword from the District President SaiRam, I offer my heartfull salutations at the lotus fee of our beloved Sarvadevatateeta Swaroopa Bhagavan Sri Sari Sathya Sai Baba Varu. The elder and chosen devotees of Krishna District were very fortunate enough in having recognized Bala Baba as God Incarnated. Swamy blessed nearly seven or eight places and devotee’s residences in Krishna Distrct. Swamy, we on behalf of thousands of devotees of Krishna District offer our salutations to you for blessing all of us to be an active member’s of esteemed organisation named after you and blessed by you. Swamy how fortunate we are when you have created a lingam and named it as Premalingam and given to Vijayawada Samithi for daily abhishekam and declared whosoever taken the abhisheka jalam will get rid of any disease whatsoever. Swamy long back during darshan time you hve enlighted all of us to concentrate on the followup of any activity in any wing by asking “machines teesukunnavallandaru bagunnaraa”. After hearing this we have been taking continuous steps for follow up whether it is medical seva, DJP or any educational activity. -
Slno DISTRICT COD E DISTRICT NAME MANDAL CODE
Secretariat Master Report For KRISHNA DISTRICT_COD DISTRICT_ MANDAL SECRETAR slno MANDAL_NAME SECRETARIAT_NAME E NAME _CODE IAT_CODE 1 106 KRISHNA 1519 10690667 MADHAVARAM 2 106 KRISHNA 1519 10690665 KRISHNARAOPALEM 3 106 KRISHNA 1519 10690661 GOLLAMANDLA 4 106 KRISHNA 1519 10690659 A.KONDURUTHANDA 5 106 KRISHNA 1519 10690660 CHEEMALAPADU 6 106 KRISHNA 1519 10690668 POLLOSETTIPADI 7 106 KRISHNA 1519 10690662 KHAMBHAMPADU1 A.KONDURU 8 106 KRISHNA 1519 10690658 A.KONDURU 9 106 KRISHNA 1519 10690669 RAMCHANDRAPURAM 10 106 KRISHNA 1519 10690670 REPUDI 11 106 KRISHNA 1519 10690664 KODURU 12 106 KRISHNA 1519 10690666 KUMMARIKUNTLA 13 106 KRISHNA 1519 10690663 KHAMBHAMPADU2 14 106 KRISHNA 1519 10690671 VALLAMPATLA 15 106 KRISHNA 1509 10690457 ADAVINEKKALAM1 16 106 KRISHNA 1509 10690468 KOTHAEDARA 17 106 KRISHNA 1509 10690475 VATTIGUDIPADU 18 106 KRISHNA 1509 10690459 AGIRIPALLI1 19 106 KRISHNA 1509 10690463 CHOPPARMETLA 20 106 KRISHNA 1509 10690473 SURAVRAM 21 106 KRISHNA 1509 10690460 AGIRIPALLI2 22 106 KRISHNA 1509 10690474 VADLAMANU 23 106 KRISHNA 1509 10690467 KANASANAPALLI 24 106 KRISHNA 1509 AGIRIPALLI 10690462 CHINNAAGIRIPALLI 25 106 KRISHNA 1509 10690466 EDULAGUDEM 26 106 KRISHNA 1509 10690464 EDARA1 27 106 KRISHNA 1509 10690465 EDARA2 28 106 KRISHNA 1509 10690469 KRISHNAVARUM 29 106 KRISHNA 1509 10690472 S.A.PETA 30 106 KRISHNA 1509 10690458 ADAVINEKKALAM2 31 106 KRISHNA 1509 10690461 BODDANAPALLI 32 106 KRISHNA 1509 10690470 NARASINGAPALEM 33 106 KRISHNA 1509 10690471 NUGONDAPALLI 34 106 KRISHNA 1497 10690267 ASWARAPALEM 35 106 KRISHNA