Introduction to the Endocrine System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
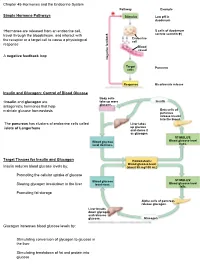
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low Ph in Duodenum
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low pH in duodenum •Hormones are released from an endocrine cell, S cells of duodenum travel through the bloodstream, and interact with secrete secretin ( ) Endocrine the receptor or a target cell to cause a physiological cell response Blood vessel A negative feedback loop Target Pancreas cells Response Bicarbonate release Insulin and Glucagon: Control of Blood Glucose Body cells •Insulin and glucagon are take up more Insulin antagonistic hormones that help glucose. maintain glucose homeostasis Beta cells of pancreas release insulin into the blood. The pancreas has clusters of endocrine cells called Liver takes islets of Langerhans up glucose and stores it as glycogen. STIMULUS: Blood glucose Blood glucose level level declines. rises. Target Tissues for Insulin and Glucagon Homeostasis: Blood glucose level Insulin reduces blood glucose levels by: (about 90 mg/100 mL) Promoting the cellular uptake of glucose Blood glucose STIMULUS: Slowing glycogen breakdown in the liver level rises. Blood glucose level falls. Promoting fat storage Alpha cells of pancreas release glucagon. Liver breaks down glycogen and releases glucose. Glucagon Glucagon increases blood glucose levels by: Stimulating conversion of glycogen to glucose in the liver Stimulating breakdown of fat and protein into glucose Diabetes Mellitus Type I diabetes mellitus (insulin-dependent) is an autoimmune disorder in which the immune system destroys pancreatic beta cells Type II diabetes -

Biology F – Lesson 4 – Endocrine & Reproductive Systems
Unit: Biology F – Endocrine & Reproduction LESSON 4.1 - AN INTRODUCTION TO THE ENDOCRINE SYSTEM Overview: Students research the location and function of the major endocrine glands and consider some endocrine system disorders. Suggested Timeline: 1 hour Materials: • An Introduction to the Endocrine System (Student Handout) • QUIZ – The Endocrine System (Student Handout) • student access to computers with the internet Method: 1. Allow students to use computers with internet access and other resources from the library to complete their questions on the endocrine system. 2. Students write quiz on the endocrine system. Assessment and Evaluation: • Affective assessment of student understanding of parts of the endocrine system • Student grade on quiz Extension: Assign a student or group of students to ‘be’ a specific endocrine gland. Have them role play for the class to get the idea of the function of the gland across to their classmates. Science 21 Bio F – Endocrine B152 Unit: Biology F – Endocrine & Reproduction Student Handout Name: ______________ Partner(s): _________________________ Date: ____________ Period: _____ An Introduction to the Endocrine System Go to the following website: http://health.howstuffworks.com/adam-200091.htm Watch the video to complete the following questions. 1. The endocrine system is composed primarily of _________________ that produce chemical messengers called __________________. 2. List the names of glands of the endocrine system, as mentioned in the video. Hint: There are 8 in total. 3. The endocrine and ________________ systems work closely together. The brain sends info to the endocrine glands and the endocrine glands send feedback to the brain. 4. The part of the brain that is known as the ‘master switchboard’ and controls the endocrine system is called the _____________________. -

Endocrine Pathology Crines… Molecular Signaling Endocrine Pathology Endocrine Pathology
Endocrine Pathology Crines… Molecular signaling Autocrine Paracrine Endocrine Endocrine Pathology Endocrine Pathology Cell signaling system Too much hormone activity Surface receptors Too little hormone activity cAMP and tyrosine kinase system Autoimmune destruction Cytoplasmic receptors Inflammatory destruction Penetrate cell membrane Tumor or vascular destruction Gene activation -> transcription -> translation Space occupying lesions (tumors) Intranuclear receptors Malignant Gene activation -> transcription -> translation Benign 1 Endocrine Pathology Endocrine Pathology All parts of the endocrine system interconnect. All parts of the endocrine system interconnect Pituitary Pathology Too much Too little Especially space occupying lesions The Basics Pituitary Vascular Signaling proteins Anterior are release in hypothalmus. Comes from GI Travel by blood to Controlled by hypothalmus anterior pituitary Cause release of Posterior many activating Hormones orginate hormones further up. System of amplification 2 Pituitary Control Space Occupying Lesions Tumors Embryonic rests Squeeze gland out of existence. Generalized failure Visual field changes Visual Fields Loss of temporal fields. Nasal retina Damage to decusating optic nerve fibers 3 Acromegaly Pituitary Adenomas Rare Growth hormone excess after closing Make nothing or of epiphyses. Prolactin Periosteal bone ACTH, GH,TSH are very rare growth. More often end up with pituitary Diabetes failure. Prognathism Squeeze the daylights out of the -

Chapter 20: Endocrine System
EndocrineEndocrine SystemSystem Modified by M. Myers 1 TheThe EndocrineEndocrine SystemSystem 2 EndocrineEndocrine GlandsGlands z The endocrine system is made of glands & tissues that secrete hormones. z Hormones are chemicals messengers influencing a. metabolism of cells b. growth and development c. reproduction, d. homeostasis. 3 HormonesHormones Hormones (chemical messengers) secreted into the bloodstream and transported by blood to specific cells (target cells) Hormones are classified as 1. proteins (peptides) 2. Steroids 4 HormoneHormone ClassificationClassification z Steroid Hormones: – Lipid soluble – Diffuse through cell membranes – Endocrine organs z Adrenal cortex z Ovaries z Testes z placenta 5 HormoneHormone ClassificationClassification z Nonsteroid Hormones: – Not lipid soluble – Received by receptors external to the cell membrane – Endocrine organs z Thyroid gland z Parathyroid gland z Adrenal medulla z Pituitary gland z pancreas 6 HormoneHormone ActionsActions z “Lock and Key” approach: describes the interaction between the hormone and its specific receptor. – Receptors for nonsteroid hormones are located on the cell membrane – Receptors for steroid hormones are found in the cell’s cytoplasm or in its nucleus 7 http://www.wisc- online.com/objects/index_tj.asp?objID=AP13704 8 EndocrineEndocrine SystemSystem z There is a close assoc. b/w the endocrine & nervous systems. z Hormone secretion is usually controlled by either negative feedback or antagonistic hormones that oppose each other’s actions 9 HypothalamusHypothalamus 1. regulates the internal environment through the autonomic system 2. controls the secretions of the pituitary gland. 10 HypothalamusHypothalamus && PituitaryPituitary GlandGland posteriorposterior pituitary/pituitary/ anterioranterior pituitarypituitary 11 PosteriorPosterior PituitaryPituitary The posterior pituitary secretes zantidiuretic hormone (ADH) zoxytocin 12 13 14 AnteriorAnterior pituitarypituitary glandgland 1. -

11 Hormonal Coordination Table 1 the Main Roles of Hormones Produced by the Different Endocrine Glands
B 11 Hormonal ■ B11 Hormonal coordination Table 1 The main roles of hormones produced by the different endocrine glands Endocrine gland Role of the hormones coordination Pituitary Controls growth in children Stimulates the thyroid gland to make thyroxine to control the rate of metabolism 11.1 Principles of hormonal control In women – stimulates the ovaries to produce and release eggs and make the female sex hormone oestrogen Learning objectives In Chapter B 10 you discovered how the nervous system acts to coordinate In men – stimulates the testes to make sperm and the male sex After this topic, you should know: and control your body, reacting in seconds to changes in your internal and hormone testosterone external environments. However, it is very important that your body acts as Thyroid Controls the metabolic rate of the body ● what a hormone is a coordinated whole, not just from minute to minute but from day to day Pancreas Controls the levels of glucose in the blood Figure 2 It isn’t just humans who need ● the main organs of the endocrine and year to year throughout your life. You have a second coordination and hormones – without the hormones from system Adrenal Prepares the body for stressful situations – ‘fight or flight’ response control system to help with this – the endocrine system. their thyroid glands, these tadpoles will ● the role of the pituitary gland. Ovaries Controls the development of the female secondary sexual characteristics and is involved in the menstrual cycle never become frogs The endocrine system Testes Controls the development of the male secondary sexual The endocrine system is made up of glands that secrete chemicals called characteristics and is involved in the production of sperm hormones directly into the bloodstream. -

Endocrine System with Special Reference to Thyroid Gland
Odisha Review December - 2012 Endocrine System With Special Reference to Thyroid Gland Soma Mishra Endocrine system consisting of a group of ductless glands viz. pituitary, thyroid, parathyroid, pineal, thymus, gonads, pancreas, adrenal etc. plays a very vital role in governing human behavior. Thyroid is one of the most important glands that control body’s metabolism and calcium level. It secretes iodothyronines that are (tri-iodo- thyronine, thyroxine) and calcitonin. Its secretion is mainly regulated by TRH (thyrotropin releasing hormone) and TSH (thyroid stimulating hormone). It helps in growth (physical, sexual, mental) – development- metamorphosis and calorigenesis- metabolism. The status of thyroid gland may be Euthyroid or Hypothyroid or Hyperthyroid. Hypothyroidism includes cretinism in children and myxoedema in adults. Common causes of hyperthyroid state are Grave’s disease, multinodular goiter, thyroiditis, etc. Any enlargement of thyroid gland, regardless of cause, is called goiter. Some common investigations for Introduction thyroid diseases are estimation of serum T3, T4 The endocrine system or hormonal system and TSH, cholesterol, radioiodine uptake, thyroid is a complex system composed of a group of imaging, etc. Common drug used in ductless glands known as endocrine glands that hypothyroidism is eltroxin, hyperthyroidism is pour their secretions i.e. hormones directly into carbimazole and iodine supplementation in goiter. blood for passage to different body organs known This paper presents a full picture of thyroid gland, as target organs in order to control their its functioning, disorders, and treatments which is functioning, metabolism, cell permeability, growth, very significant for human survival. differentiation and stress conditions. 54 December - 2012 Odisha Review The endocrine system includes the Diseases of the endocrine system result pituitary gland, thyroid gland, parathyroid glands, from too much or too little hormone secretion or adrenal gland, pancreas, ovaries and testes. -

210825 Endocrine System 5 – the Function of the Pineal and Thymus
Copyright EMAP Publishing 2021 This article is not for distribution except for journal club use Clinical Practice Keywords Endocrine system/ Hormones/Pineal gland/Thymus gland Systems of life This article has been Endocrine system double-blind peer reviewed In this article... ● Endocrine functions of the pineal and thymus glands ● Hormones secreted by the pineal and thymus glands ● Role of the pineal and thymus glands in mediating essential physiological processes Endocrine system 5: the function of the pineal and thymus glands Key points Authors John Knight is associate professor in biomedical science; Zubeyde Bayram- The pineal gland is Weston is senior lecturer in biomedical science; Maria Andrade is honorary associate a neuroendocrine professor in biomedical science; all at College of Human and Health Sciences, gland in the brain, Swansea University. which produces the hormone melatonin Abstract The endocrine system consists of glands and tissues that produce and secrete hormones to regulate and coordinate vital bodily functions. This article, the Melatonin is key to fifth in an eight-part series on the endocrine system, explores the anatomy and synchronising the physiology of the pineal and thymus glands, and describes how they regulate and body’s biological coordinate vital physiological processes in the body through hormonal action. rhythms and has an influence on Citation Knight J et al (2021) Endocrine system 5: pineal and thymus glands. immunity Nursing Times [online]; 117: 9, 54-58. The thymus has both immune and he endocrine system comprises with a rich blood flow of around 4ml/ endocrine functions, glands and tissues that produce min/g, second only to that of the kidneys. -

The Endocrine System from Our Neurotransmitter Chapter: Endocrine Neurotransmitters Convey a Message from the Glands Secrete Sending Neuron to the Receiving Neuron(S)
11/10/2014 The Endocrine System From Our Neurotransmitter Chapter: Endocrine Neurotransmitters convey a message from the glands secrete sending neuron to the receiving neuron(s). “hormones” Hormones, on the other hand, “broadcast” a message directly into throughout the body via the bloodstream, so are able bloodstream. to influence cells in many distant organs/tissues. But we’ll hold off on hormones for the moment, In Chap 11 the because before there could be hormones, there had Hypothalamus, to be genes to develop the glands. Pituitary, Gonads, and Adrenal glands will play a role in sexuality What defines male or female ? Sexual Differentiation in Mammals (Chap 11) How do we become the sexual individuals we are? Differentiation Role of Sex Chromosomes • Genetic sex (XX or XY) is determined by the sperm (X-bearing or Y-bearing) that fertilizes the egg. • Early gonads have potential to be either ovaries or testes for ~6 weeks. • Sex-determining region of the Y chromosome (SRY) is a gene producing a *protein causing the middle of baby gonads to become testes. • If testes develop, they begin to produce androgens like testosterone. • If SRY gene is not present, the outside of the early gonads turns into ovaries. *sometimes called testis-determining factor 1 11/10/2014 Experimental Evidence • Removal of SRY gene from Y XY mouse develops as a female • Add SRY gene to X XX mouse develops as a male • Injection of SRY’s protein in genetic female develops testes • Inject genetic male with drug that blocks the SRY’s protein develops ovaries Figure 13.6 Endocrine Glands – Release hormones directly into bloodstream Although the pituitary is sometimes called the “master gland” in Organizational Effects fact the hypothalamus is the “master” of the pituitary. -

Endocrine System 4: Adrenal Glands
Copyright EMAP Publishing 2021 This article is not for distribution except for journal club use Clinical Practice Keywords Endocrine system/ Hormones/Adrenal glands Systems of life This article has been Endocrine system double-blind peer reviewed In this article... ● Endocrine functions of the adrenal glands ● Hormones of the adrenal glands ● The role of adrenal gland hormones in mediating essential physiological processes Endocrine system 4: adrenal glands Key points Authors Maria Andrade is honorary associate professor in biomedical science; There are two Zubeyde-Bayram Weston is senior lecturer in biomedical science; John Knight is adrenal glands: one associate professor in biomedical science; all at College of Human and Health located above each Sciences, Swansea University. kidney NT SELF- Abstract The endocrine system consists of glands and tissues that produce and ASSESSMENT Adrenal glands secrete hormones to regulate and coordinate vital bodily functions. This article, the Test your consist of two parts, fourth in an eight-part series on the endocrine system, explores the anatomy and knowledge. the cortex and the physiology of the adrenal glands, and describes how they regulate and coordinate After reading this medulla, which each vital physiological processes in the body through hormonal action. article go to produce different nursingtimes.net/ NTSAAdrenal hormones Citation Andrade M et al (2021) Endocrine system 4: adrenal glands. Nursing Times If you score 80% [online]; 117: 8, 54-58. or more, you will The adrenal cortex receive a certificate produces a diverse that you can use range of steroid as revalidation his eight-part series on the endo- colour and are positioned retroperito- evidence. -

1 General Overview of the Endocrine System Questions to Be Thinking
General Overview of the Endocrine System Questions to be thinking about to help organize your learning of the components of the Endocrine System: Anatomy 1) Where are the different hormone producing cells located? 2) What hormone does a particular type of endocrine cell or neuron produce? General Overview of the Endocrine System Questions to be thinking about to help organize your learning of the components of the Endocrine System: Hormones 1) What is their general chemical structure? 2) How does their structure affect their function? 3) Where are they produced? 4) What environmental or physiological stimuli regulate their production and secretion? 5) Is their production and secretion under direct neural or hormonal control? 6) What are their targets? 7) What effects do they produce on their targets? 8) How do they produce their effects on their targets (how is the signal transduced? i.e. receptor mechanisms) 1 Often endocrine cells are clumped together into a well defined gland (e.g. pituitary, thyroid, adrenal, testes, ovaries), but not always (e.g. gut, liver, lung). Often endocrine cells are clumped together into a well defined gland (e.g. pituitary, thyroid, adrenal, testes, ovaries), but not always (e.g. gut, liver, lung). Remember, it's cells that produce hormones, not glands. Although many glands secrete more than one type of hormone, most neurons or endocrine cells only produce one type of hormone (there are a few exceptions). 2 Some Terminology Neurohormone: a hormone that is produced by a neuron Some Terminology All of the hormones we discuss can be classified as belonging to one of 2 general functional categories: 1] Releasing hormone (or factors) — hormone that acts on endocrine cells to regulate the release of other hormones. -

Endocrine System
Human Body Series Endocrine System Quiz Answer Key 1. List five functions of the endocrine system: Any five of the following: regulating mood, growth and development; tissue function; the fight or flight response; metabolism; blood glucose levels; sexual function and reproductive processes. 2. In general, the endocrine system is in charge of body processes that happen slowly, such as cell growth. Faster processes like breathing and body movement are controlled by the nervous system . 3. The brain contains these three glands: pituitary gland, hypothalamus , pineal gland (or pineal body) . 4. Hormones are chemical messengers that transfer information and instructions from one set of cells to another. 5. Once a hormone is secreted, it travels from the endocrine gland that produced it through the bloodstream to the cells designed to receive its message. These cells are called target cells . 6. The pancreas produces two important hormones, insulin and glucagon . 7. If the pancreas doesn’t produce enough insulin, the result is diabetes . 8. Parathyroid hormone controls the level of calcium in the blood. 9. The thyroid gland is involved in metabolism , the process by which the fuel in the food we eat is converted into cellular energy. 10. When hormone levels reach a certain normal amount in the blood, the endocrine system has a built-in turnoff process. It is called negative feedback system . 11. Endocrine glands release more than 20 major hormones directly into the bloodstream. 12. The pineal gland secretes melatonin . 13. If the pituitary glands release hormones that stimulate the gonads to produce sex hormones too early, some kids may experience precocious puberty and begin to go through puberty at a very young age. -

The Endocrine System Dr
The Endocrine System Dr. Ali Ebneshahidi Copyright © 2006 Pearson Education, Inc., publishing as Benjamin Cummings Endocrine System . The endocrine system interacts with the nervous system to coordinate and integrate body activities by means of hormones . Endocrine tissues and organs secrete hormone into body fluids (mainly blood and lymph) directly using diffusion. Exocrine tissues, such as salivary glands, and sebaceous glands, secrete chemical substances through ducts into an open space. Copyright © 2006 Pearson Education, Inc., publishing as Benjamin Cummings Five major functions of hormones . a) Regulate metabolic processes (e.g. thyroid hormones). b) Control the rate of chemical reactions (e.g. growth hormone). c) Aid in the transport of substances across the cell membrane of target cells (e.g. insulin and glucagon). d) Regulate water and electrolyte balances (e.g. antidiurectic hormone, calcitonin, and aldosterone). e) Play a vital role in reproduction, growth and development (e.g. estrogens , progesterone, and testosterone). Copyright © 2006 Pearson Education, Inc., publishing as Benjamin Cummings Major Endocrine Organs Copyright © 2006 Pearson Education, Inc., publishing as Benjamin Cummings Chemistry of Hormones . Hormones are organic compounds secreted by endocrine glands, that have a potent effect in target cells Two types of hormones: . a) Protein hormones: made of amino acids joined by peptide bonds. fat – insoluble; as a result cannot diffuse across the membrane of target cells . most hormones belong to this group except hormones secreted by the gonads (testis and ovary) and the adrenal cortex. b) Steroid hormones: made of fatty acids using cholesterol as a functional group. Fat-soluble; as a result can diffuse into target cells .