Some Effects of Calcium Ions on the Action Potential of Single Nodes of Ranvier
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Facilitation of Rabbit ב1B Calcium Channels: Involvement of Endogenous Gגד Subunits
Keywords: Calcium channel, G protein, Facilitation 7535 Journal of Physiology (1998), 509.1, pp.15—27 15 Facilitation of rabbit á1B calcium channels: involvement of endogenous Gâã subunits Gary J. Stephens, Nicola L. Brice, Nicholas S. Berrow and Annette C. Dolphin Department of Pharmacology, University College London and Royal Free Hospital School of Medicine, London WC1E 6BT, UK (Received 30 October 1997; accepted after revision 26 January 1998) 1. The á1B (N-type) calcium channel shows strong G protein modulation in the presence of G protein activators or Gâã subunits. Using transient expression in COS_7 cells of á1B together with the accessory subunits áµ—ä and â2a,wehaveexaminedtheroleofendogenous Gâã subunits in the tonic modulation of á1B, and compared this with modulation by exogenously expressed Gâã subunits. 2. Prepulse facilitation of control á1BÏáµ—äÏâ2a currents was always observed. This suggests the existenceoftonicmodulationofá1B subunits. To determine whether endogenous Gâã is involved in the facilitation observed in control conditions, the âARK1 Gâã-binding domain (amino acids 495—689) was overexpressed, in order to bind free Gâã subunits. The extent of control prepulse-induced facilitation was significantly reduced, both in terms of current amplitude and the rate of current activation. In agreement with this, GDPâS also reduced the control facilitation. 3. Co-expression of the GâÔãµ subunit, together with the á1BÏáµ—äÏâ2a calcium channel combination, resulted in a marked degree of depolarizing prepulse-reversible inhibition of the whole-cell ICa or IBa. Both slowing of current activation and inhibition of the maximum current amplitude were observed, accompanied by a depolarizing shift in the mid-point of the voltage dependence of activation. -

7.016 Introductory Biology Fall 2018
7.016: Fall 2018: MIT 7.016 Recitation 15 – Fall 2018 (Note: The recitation summary should NOT be regarded as the substitute for lectures) (This material is COPYRIGHT protected.) Summary of Lecture 22 (11/2): Neurons and action potentials: Ions can move across membranes through pumps and channels. Integral membrane proteins like these cross the membrane via transmembrane domains. Pumps are ATPases that set up the concentration gradients of ions across cell membranes, such that K+ is high inside cells and other ions (such as Cl–, Na+, and Ca2+) are high outside cells. A membrane potential is only set up by ions that move freely across the membrane through open channels, creating one side of the membrane that is more positive relative to the other side. This movement of ions does not dissipate the concentration gradient because the number of ions that move to generate a membrane potential is very small compared to the number of ions that need to be pumped to create a concentration gradient. Most cell membranes only contain open K+ channels and thus only K+ flows freely across membranes through channels; K+ is high inside, causing it to flow outside, giving the inside of cells a negative membrane potential. Ions can move across the membrane through open ion channels. Two forces act to dictate this movement – the concentration gradient and the electrical gradient. Ions move down their concentration gradient through channels, and ions move towards the side of the membrane that harbors the opposite charge. Neurons are the cells of your nervous system that make connections with each other to transmit impulse/ signals. -

An Unexpected Role for Brain-Type Sodium Channels in Coupling of Cell Surface Depolarization to Contraction in the Heart
An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart Sebastian K. G. Maier*, Ruth E. Westenbroek*, Kenneth A. Schenkman†, Eric O. Feigl‡, Todd Scheuer*, and William A. Catterall*§ Departments of *Pharmacology, †Pediatrics, and ‡Physiology and Biophysics, University of Washington, Seattle, WA 98195 Contributed by William A. Catterall, December 27, 2001 ␣ Voltage-gated sodium channels composed of pore-forming and ventricular myocytes compared with Nav1.5, but they contribute auxiliary  subunits are responsible for the rising phase of the significantly to coupling of cell surface depolarization to con- action potential in cardiac muscle, but the functional roles of traction because of their location in the transverse tubules. Our distinct sodium channel subtypes have not been clearly defined. results provide the first evidence, to our knowledge, for a unique Immunocytochemical studies show that the principal cardiac pore- functional role of TTX-sensitive brain-type sodium channels in forming ␣ subunit isoform Nav1.5 is preferentially localized in the heart. intercalated disks, whereas the brain ␣ subunit isoforms Nav1.1, Nav1.3, and Nav1.6 are localized in the transverse tubules. Sodium Methods currents due to the highly tetrodotoxin (TTX)-sensitive brain iso- All procedures were approved by the Institutional Animal Care forms in the transverse tubules are small and are detectable only and Use Committee of the University of Washington. after activation with  scorpion toxin. Nevertheless, they play an important role in coupling depolarization of the cell surface mem- Cell Isolation. Ventricular myocytes were isolated from adult male brane to contraction, because low TTX concentrations reduce left (8–10 weeks) wild-type B6129F1 mice, as described (14). -

Genetic Alteration of the Metal/Redox Modulation of Cav3.2 T-Type
Genetic alteration of the metal/redox modulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability Tiphaine Voisin, Emmanuel Bourinet, Philippe Lory To cite this version: Tiphaine Voisin, Emmanuel Bourinet, Philippe Lory. Genetic alteration of the metal/redox mod- ulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability. The Journal of Physiology, Wiley, 2016, 594 (13), pp.3561–74. 10.1113/JP271925. hal-01940961 HAL Id: hal-01940961 https://hal.archives-ouvertes.fr/hal-01940961 Submitted on 30 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. J Physiol 594.13 (2016) pp 3561–3574 3561 Genetic alteration of the metal/redox modulation of Cav3.2 T-type calcium channel reveals its role in neuronal excitability Tiphaine Voisin1,2,3, Emmanuel Bourinet1,2,3 and Philippe Lory1,2,3 1Centre National pour la Recherche Scientifique UMR 5203, D´epartement de Physiologie, Institut de G´enomique Fonctionnelle, Universit´edeMontpellier, Montpellier, F-34094 France 2Institut National de la Sant´eetdelaRechercheM´edicale, U 1191, Montpellier, F-34094 France Neuroscience 3LabEx ‘Ion Channel Science and Therapeutics’, Montpellier, F-34094 France Key points r In this study, we describe a new knock-in (KI) mouse model that allows the study of the H191-dependent regulation of T-type Cav3.2 channels. -

The Role of S4 Charges in Voltage-Dependent
ARTICLE The Role of S4 Charges in Voltage-dependent and Voltage-independent KCNQ1 Potassium Channel Complexes Gianina Panaghie and Geoffrey W. Abbott Greenberg Division of Cardiology, Department of Medicine and Department of Pharmacology, Cornell University, Weill Medical College, New York, NY 10021 Voltage-gated potassium (Kv) channels extend their functional repertoire by coassembling with MinK-related peptides (MiRPs). MinK slows the activation of channels formed with KCNQ1 α subunits to generate the voltage- dependent IKs channel in human heart; MiRP1 and MiRP2 remove the voltage dependence of KCNQ1 to generate potassium “leak” currents in gastrointestinal epithelia. Other Kv α subunits interact with MiRP1 and MiRP2 but without loss of voltage dependence; the mechanism for this disparity is unknown. Here, sequence alignments re- vealed that the voltage-sensing S4 domain of KCNQ1 bears lower net charge (+3) than that of any other eukaryotic voltage-gated ion channel. We therefore examined the role of KCNQ1 S4 charges in channel activation using alanine-scanning mutagenesis and two-electrode voltage clamp. Alanine replacement of R231, at the N-terminal side of S4, produced constitutive activation in homomeric KCNQ1 channels, a phenomenon not observed with previous single amino acid substitutions in S4 of other channels. Homomeric KCNQ4 channels were also made constitutively active by mutagenesis to mimic the S4 charge balance of R231A-KCNQ1. Loss of single S4 charges at positions R231 or R237 produced constitutively active MinK-KCNQ1 channels and increased the constitutively active component of MiRP2-KCNQ1 currents. Charge addition to the CO2H-terminal half of S4 eliminated constitu- tive activation in MiRP2-KCNQ1 channels, whereas removal of homologous charges from KCNQ4 S4 produced constitutively active MiRP2-KCNQ4 channels. -

Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M
Electrical Activity of the Heart: Action Potential, Automaticity, and Conduction 1 & 2 Clive M. Baumgarten, Ph.D. OBJECTIVES: 1. Describe the basic characteristics of cardiac electrical activity and the spread of the action potential through the heart 2. Compare the characteristics of action potentials in different parts of the heart 3. Describe how serum K modulates resting potential 4. Describe the ionic basis for the cardiac action potential and changes in ion currents during each phase of the action potential 5. Identify differences in electrical activity across the tissues of the heart 6. Describe the basis for normal automaticity 7. Describe the basis for excitability 8. Describe the basis for conduction of the cardiac action potential 9. Describe how the responsiveness relationship and the Na+ channel cycle modulate cardiac electrical activity I. BASIC ELECTROPHYSIOLOGIC CHARACTERISTICS OF CARDIAC MUSCLE A. Electrical activity is myogenic, i.e., it originates in the heart. The heart is an electrical syncitium (i.e., behaves as if one cell). The action potential spreads from cell-to-cell initiating contraction. Cardiac electrical activity is modulated by the autonomic nervous system. B. Cardiac cells are electrically coupled by low resistance conducting pathways gap junctions located at the intercalated disc, at the ends of cells, and at nexus, points of side-to-side contact. The low resistance pathways (wide channels) are formed by connexins. Connexins permit the flow of current and the spread of the action potential from cell-to-cell. C. Action potentials are much longer in duration in cardiac muscle (up to 400 msec) than in nerve or skeletal muscle (~5 msec). -

ACTX-Hv1c, Isolated from the Venom of the Blue Mountains
S.J. Gunning et al. Janus-faced atracotoxins block KCa channels The Janus-faced atracotoxins are specific blockers of invertebrate KCa channels Simon J. Gunning1, Francesco J. Maggio2,4, Monique J. Windley1, Stella M. Valenzuela1, Glenn F. King3 and Graham M. Nicholson1 1 Neurotoxin Research Group, Department of Medical & Molecular Biosciences, University of Technology, Sydney, Broadway, NSW 2007, Australia 2 Department of Molecular, Microbial & Structural Biology, University of Connecticut School of Medicine, Farmington, Connecticut 06032, USA and 3 Division of Chemical and Structural Biology, Institute for Molecular Bioscience, University of Queensland, Brisbane QLD 4072, Australia 4 Current affiliation: Bristol-Myers Squibb, 6000 Thompson Road, Syracuse NY 13057, USA Subdivision: Molecular Neurobiology Abbreviations: ACTX, atracotoxin; 4-AP, 4-aminopyridine; BKCa channel, 2+ + 2+ large-conductance Ca -activated K channel; CaV channel, voltage-activated Ca channel; ChTx, charybdotoxin; DRG, dorsal root ganglia; DUM, dorsal unpaired median; EGTA, ethylene glycol-bis(b-aminoethyl ether)-N,N,N’N’-tetraacetic acid; IbTx, + iberiotoxin; J-ACTX, Janus-faced atracotoxin; KA channel, transient ‘A-type’ K channel; 2+ + + KCa channel, Ca -activated K channel; KDR channel, delayed-rectifier K channel; KV + + channel, voltage-activated K channel; NaV channel, voltage-activated Na channel; Slo, Slowpoke; TEA, tetraethylammonium; TTX, tetrodotoxin. RUNNING TITLE: Janus-faced atracotoxins block KCa channels ADDRESS CORRESPONDENCE TO: Graham M. Nicholson, Department of Medical & Molecular Biosciences, University of Technology, Sydney, PO Box 123, Broadway NSW 2007, Australia. Tel: +61 2 9514-2230 Fax: +61 2 9514-2228; E-mail: [email protected] 1 S.J. Gunning et al. Janus-faced atracotoxins block KCa channels ABSTRACT The Janus-faced atracotoxins are a unique family of excitatory peptide toxins that contain a rare vicinal disulfide bridge. -

Uhm Phd 9118021 R.Pdf
INFORMATION TO USERS The most advanced technology has been used to photograph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. U·M·I University Microfilms International A Bell & Howell Information Company 300 North Zeeb Road. Ann Arbor. M148106·1346 USA 313/761-4700 800/521-0600 Order Number 9118021 Sodium channel activation mechanisms: Insights from deuterium oxide and ddta-9-tetrahydrocannabinol substitution Alicata, Daniel Andrew, Ph.D. -
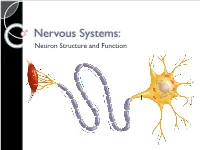
4-Nervous-System-Structure-PPT.Pdf
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation and coordination that produce coherency and result in harmonious function. Integration Cellular integration = processes within cells Whole-animal integration = selective combination and processing of sensory, endocrine, and central nervous system (CNS) information in ways that promote harmonious functioning of the whole organism within its environment. ◦ This includes its all its cells, tissues, and organs Integration Nerve cells are specialized for control and coordination. Integration ensures that an animal’s responses are smooth and coordinated rather than clashing or disjointed. Excitable Cells Neurons are a type of excitable cell ◦ Specially adapted to generate an electrical signal Can rapidly alter their membrane potential in response to an incoming signal. Vary in structure and function but use the same basic mechanism to send signals. Neuron Function – Main Points Specialized for processing and conveying information. Information is coded into changes in electrical potential across cell membranes. Neurons use action potentials to transmit these signals across long distances. Neurons allow animals to sense and respond to their environment. Benefits of Neurons Plants (no neurons): Action potentials travel @ 1-3 cm/sec Animals (neurons): Action potentials travel @ 100m/sec or 10,000cm/sec CNS to Muscles Signal Reception Dendrites & Cell Body Signal Integration Axon Hillock Signal Conduction Axon Signal Transmission Axon Terminals Signal Reception Dendrites sense and convert incoming signals into electrical signals by changing membrane potential. Cell Body = routine metabolic functions Signal Integration Incoming signals are conducted to the axon hillock If signal is sufficiently large an electrical signal, or action potential, is initiated. -

Phenytoin Inhibits the Persistent Sodium Current in Neocortical Neurons by Modifying Its Inactivation Properties
Phenytoin Inhibits the Persistent Sodium Current in Neocortical Neurons by Modifying Its Inactivation Properties Elisa Colombo1, Silvana Franceschetti1, Giuliano Avanzini1, Massimo Mantegazza1,2* 1 Department of Neurophysiopathology – Epilepsy Center, Foundation IRCCS Neurological Institute C. Besta, Milan, Italy, 2 Institut de Pharmacologie Mole´culaire et Cellulaire (IPMC), CNRS UMR7275 and University of Nice-Sophia Antipolis, Valbonne, France Abstract + The persistent Na current (INaP) is important for neuronal functions and can play a role in several pathologies, although it is + small compared to the transient Na current (INaT). Notably, INaP is not a real persistent current because it undergoes inactivation with kinetics in the order of tens of seconds, but this property has often been overlooked. Na+ channel blockers, drugs used for treating epilepsy and other diseases, can inhibit INaP, but the mechanism of this action and the conditions in which INaP can be actually inhibited have not been completely clarified yet. We evaluated the action of phenytoin (PHT), a + prototype anti-epileptic Na channel blocker, on INaP inactivation in pyramidal neurons of rat sensorimotor cortical slices at different concentrations, from 5 to 100 mM. PHT did not modify INaP evoked with depolarizing voltage ramps of 50 or 21 21 100 mVs , but decreased INaP evoked by slower voltage ramps (10 mVs ). However, at all of the tested concentrations, PHT decreased INaP evoked by faster ramps when they were preceded by inactivating pre-pulses. Moreover, PHT shifted towards negative potentials the voltage-dependence of INaP inactivation and accelerated its kinetics of development also at depolarized potentials (+40 mV), not consistently with a simple inactivated state stabilizer. -

Electrolyte Disorders and Arrhythmogenesis
Cardiology Journal 2011, Vol. 18, No. 3, pp. 233–245 Copyright © 2011 Via Medica REVIEW ARTICLE ISSN 1897–5593 Electrolyte disorders and arrhythmogenesis Nabil El-Sherif1, Gioia Turitto2 1State University of NY, Downstate Medical Center and NY Harbor VA Healthcare System, Brooklyn, NY, USA 2Methodist University Hospital, Brooklyn, NY, USA Abstract Electrolyte disorders can alter cardiac ionic currents kinetics and depending on the changes can promote proarrhythmic or antiarrhythmic effects. The present report reviews the mecha- nisms, electrophysiolgical (EP), electrocardiographic (ECG), and clinical consequences of elec- trolyte disorders. Potassium (K+) is the most abundent intracellular cation and hypokalemia is the most commont electrolyte abnormality encountered in clinical practice. The most signifcant ECG manifestation of hypokalemia is a prominent U wave. Several cardiac and + non cardiac drugs are known to suppress the HERG K channel and hence the IK, and especially in the presence of hypokalemia, can result in prolonged action potential duration and QT interval, QTU alternans, early afterdepolarizations, and torsade de pointes ventricu- lar tachyarrythmia (TdP VT). Hyperkalemia affects up to 8% of hospitalized patients mainly in the setting of compromised renal function. The ECG manifestation of hyperkalemia de- pends on serum K+ level. At 5.5–7.0 mmol/L K+, tall peaked, narrow-based T waves are seen. At > 10.0 mmol/L K+, sinus arrest, marked intraventricular conduction delay, ventricular techycardia, and ventricular fibrillation can develop. Isolated abnormalities of extracellular calcium (Ca++) produce clinically significant EP effects only when they are extreme in either direction. Hypocalcemia, frequently seen in the setting of chronic renal insufficiency, results in prolonged ST segment and QT interval while hypercalcemia, usually seen with hyperparathy- roidism, results in shortening of both intervals. -

Two Components of the Cardiac Action Potential I
Two Components of the Cardiac Action Potential I. Voltage-time course and the effect of acet.flcholine on atrial and nodal cells of the rabbit heart ANTONIO PAES de CARVALHO, BRIAN FRANCIS HOFFMAN, and MARILENE de PAULA CARVALHO From the Instituto de Biofisica da Universidade Federal do Rio de Janeiro, Rio de Janeiro (GB), Brazil, and the Department of Pharmacology, College of Physicians and Surgeons, Columbia University, New York 10032 ABSTRACT Transmembrane potentials recorded from the rabbit heart in vitro were displayed as voltage against time (V, t display), and dV/dt against voltage (I?, V or phase-plane display). Acetylcholine was applied to the record- ing site by means of a hydraulic system. Results showed that (a) differences in time course of action potential upstroke can be explained in terms of the rela- tive magnitude of fast and slow phases of depolarization; (b) acetylcholine is capable of depressing the slow phase of depolarization as well as the plateau of the action potential; and (¢) action potentials from nodal (SA and AV) cells seem to lack the initial fast phase. These results were construed to support a two-component hypothesis for cardiac electrogenesis. The hypothesis states that cardiac action potentials are composed of two distinct and physiologically separable "components" which result from discrete mechanisms. An initial fast component is a sodium spike similar to that of squid nerve. The slow com- ponent, which accounts for both a slow depolarization during phase 0 and the plateau, probably is dependent on the properties of a slow inward current hav- ing a positive equilibrium potential, coupled to a decrease in the resting potas- sium conductance.