Etection of Echinococcus Spp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Simulation of the Potential Impacts of Projected Climate Change on Streamflow in the Vakhsh River Basin in Central Asia Under CMIP5 RCP Scenarios
water Article Simulation of the Potential Impacts of Projected Climate Change on Streamflow in the Vakhsh River Basin in Central Asia under CMIP5 RCP Scenarios Aminjon Gulakhmadov 1,2,3,4 , Xi Chen 1,2,*, Nekruz Gulahmadov 1,3,5, Tie Liu 1 , Muhammad Naveed Anjum 6 and Muhammad Rizwan 5,7 1 State Key Laboratory of Desert and Oasis Ecology, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, Urumqi 830011, China; [email protected] (A.G.); [email protected] (N.G.); [email protected] (T.L.) 2 Research Center for Ecology and Environment of Central Asia, Chinese Academy of Sciences, Urumqi 830011, China 3 Institute of Water Problems, Hydropower and Ecology of the Academy of Sciences of the Republic of Tajikistan, Dushanbe 734042, Tajikistan 4 Ministry of Energy and Water Resources of the Republic of Tajikistan, Dushanbe 734064, Tajikistan 5 University of Chinese Academy of Sciences, Beijing 100049, China; [email protected] 6 Department of Land and Water Conservation Engineering, Faculty of Agricultural Engineering and Technology, Pir Mehr Ali Shah Arid Agriculture University Rawalpindi, Rawalpindi 46000, Pakistan; [email protected] 7 Key Laboratory of Remote Sensing and Geospatial Science, Northwest Institute of Eco-Environment and Resources, Chinese Academy of Sciences, Lanzhou 730000, China * Correspondence: [email protected]; Tel.: +86-136-0992-3012 Received: 1 April 2020; Accepted: 15 May 2020; Published: 17 May 2020 Abstract: Millions of people in Uzbekistan, Turkmenistan, Tajikistan, and Kyrgyzstan are dependent on the freshwater supply of the Vakhsh River system. Sustainable management of the water resources of the Vakhsh River Basin (VRB) requires comprehensive assessment regarding future climate change and its implications for streamflow. -
CBD Strategy and Action Plan
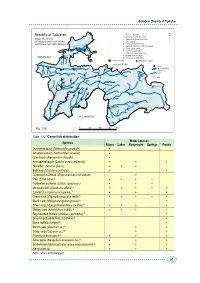
Biological Diversity of Tajikistan Republic of Tajikistan The Legend: 1 - Acipenser nudiventris Lovet 2 - Salmo trutta morfa fario Linne ya 3 - A.a.a. (Linne) ar rd Sy 4 - Ctenopharyngodon idella Kayrakkum reservoir 5 - Hypophthalmichtus molitrix (Valenea) Khujand 6 - Silurus glanis Linne 7 - Cyprinus carpio Linne a r a 8 - Lucioperca lucioperea Linne f s Dagano-Say I 9 - Abramis brama (Linne) reservoir UZBEKISTAN 10 -Carassus auratus gibilio Katasay reservoir economical pond distribution location KYRGYZSTAN cities Zeravshan lakes and water reservoirs Yagnob rivers Muksu ob Iskanderkul Lake Surkh o CHINA b r Karakul Lake o S b gou o in h l z ik r b e a O b V y u k Dushanbe o ir K Rangul Lake o rv Shorkul Lake e P ch z a n e n a r j V ek em Nur gul Murg u az ab s Y h k a Y u ng Sarez Lake s l ta i r a u iz s B ir K Kulyab o T Kurgan-Tube n a g i n r Gunt i Yashilkul Lake f h a s K h Khorog k a Zorkul Lake V Turumtaikul Lake ra P a an d j kh Sha A AFGHANISTAN m u da rya nj a P Fig. 1.16. 0 50 100 150 Km Table 1.12. Game fish distribution Water sources Species Rivers Lakes Reservoirs Springs Ponds Dushanbe loach (Nemachilus pardalis) + Amudarya loach (Nemachilus oxianus) + Gray loach (Nemachilus dorsalis) + Aral spined loach (Cobitis aurata aralensis) + + + Sheatfish (Sclurus glanis) + + + Bullhead (Ictalurus punctata) А + + Turkestan bullhead (Glyptosternum reticulatum) + Pike (Esox lucius) + + + + Turkestan bullhead (Cottus spinolosus) + + + Mosquito fish (Gambusia affinis) А + + + + + Zander (Lucioperca lucioperea) А + + + Grass carp (Ctenopharyngodon della) А + + + + + Black carp (Mylopharyngodon piceus) А + Silver carp (Hypophthalmichthus molitrix) А + + + + Motley carp (Aristichthus nobilis) А + + + + Big-mouthed buffalo (Ictiobus cyprinellus) А + Small-mouthed buffalo (I.bufalus) А + Black buffalo (I.niger) А + Mirror carp (Cyprinus sp.) А + + Scaly carp (Cyprinus sp.) А + + Pseudorasbora parva А + + + Amur goby (Neogobius amurensis sp.) А + + + Snakehead (Ophiocephalus argus warpachowski) А + + + Hemiculter sp. -

The Geodynamics of the Pamir–Punjab Syntaxis V
ISSN 00168521, Geotectonics, 2013, Vol. 47, No. 1, pp. 31–51. © Pleiades Publishing, Inc., 2013. Original Russian Text © V.S. Burtman, 2013, published in Geotektonika, 2013, Vol. 47, No. 1, pp. 36–58. The Geodynamics of the Pamir–Punjab Syntaxis V. S. Burtman Geological Institute, Russian Academy of Sciences, Pyzhevskii per. 7, Moscow, 119017 Russia email: [email protected] Received December 19, 2011 Abstract—The collision of Hindustan with Eurasia in the Oligocene–early Miocene resulted in the rear rangement of the convective system in the upper mantle of the Pamir–Karakoram margin of the Eurasian Plate with subduction of the Hindustan continental lithosphere beneath this margin. The Pamir–Punjab syn taxis was formed in the Miocene as a giant horizontal extrusion (protrusion). Extensive nappes developed in the southern and central Pamirs along with deformation of its outer zone. The Pamir–Punjab syntaxis con tinued to form in the Pliocene–Quaternary when the deformed Pamirs, which propagated northward, were being transformed into a giant allochthon. A fold–nappe system was formed in the outer zone of the Pamirs at the front of this allochthon. A geodynamic model of syntaxis formation is proposed here. DOI: 10.1134/S0016852113010020 INTRODUCTION Mujan, BandiTurkestan, Andarab, and Albruz– The tectonic processes that occur in the Pamir– Mormul faults (Fig. 1). Punjab syntaxis of the Alpine–Himalayan Foldbelt The Pamir arc is more compressed as compared and at the boundary of this syntaxis with the Tien Shan with the Hindu Kush–Karakoram arc. Disharmony of have attracted the attention of researchers for many these arcs arose in the western part of the syntaxis due years [2, 7–9, 13, 15, 28]. -

The Amu Darya River – a Review
AMARTYA KUMAR BHATTACHARYA and D. M. P. KARTHIK The Amu Darya river – a review Introduction Source confluence Kerki he Amu Darya, also called the Amu river and elevation 326 m (1,070 ft) historically known by its Latin name, Oxus, is a major coordinates 37°06'35"N, 68°18'44"E T river in Central Asia. It is formed by the junction of the Mouth Aral sea Vakhsh and Panj rivers, at Qal`eh-ye Panjeh in Afghanistan, and flows from there north-westwards into the southern remnants location Amu Darya Delta, Uzbekistan of the Aral Sea. In ancient times, the river was regarded as the elevation 28 m (92 ft) boundary between Greater Iran and Turan. coordinates 44°06'30"N, 59°40'52"E In classical antiquity, the river was known as the Oxus in Length 2,620 km (1,628 mi) Latin and Oxos in Greek – a clear derivative of Vakhsh, the Basin 534,739 km 2 (206,464 sq m) name of the largest tributary of the river. In Sanskrit, the river Discharge is also referred to as Vakshu. The Avestan texts too refer to 3 the river as Yakhsha/Vakhsha (and Yakhsha Arta (“upper average 2,525 m /s (89,170 cu ft/s) Yakhsha”) referring to the Jaxartes/Syr Darya twin river to max 5,900 m 3 /s (208,357 cu ft/s) Amu Darya). The name Amu is said to have come from the min 420 m 3 /s (14,832 cu ft/s) medieval city of Amul, (later, Chahar Joy/Charjunow, and now known as Türkmenabat), in modern Turkmenistan, with Darya Description being the Persian word for “river”. -

Rapid Chromosomal Evolution in Enigmatic Mammal with XX in Both Sexes, the Alay Mole Vole Ellobius Alaicus Vorontsov Et Al., 1969 (Mammalia, Rodentia)
COMPARATIVE A peer-reviewed open-access journal CompCytogen 13(2):Rapid 147–177 chromosomal (2019) evolution in enigmatic mammal with XX in both sexes... 147 doi: 10.3897/CompCytogen.v13i2.34224 DATA PAPER Cytogenetics http://compcytogen.pensoft.net International Journal of Plant & Animal Cytogenetics, Karyosystematics, and Molecular Systematics Rapid chromosomal evolution in enigmatic mammal with XX in both sexes, the Alay mole vole Ellobius alaicus Vorontsov et al., 1969 (Mammalia, Rodentia) Irina Bakloushinskaya1, Elena A. Lyapunova1, Abdusattor S. Saidov2, Svetlana A. Romanenko3,4, Patricia C.M. O’Brien5, Natalia A. Serdyukova3, Malcolm A. Ferguson-Smith5, Sergey Matveevsky6, Alexey S. Bogdanov1 1 Koltzov Institute of Developmental Biology, Russian Academy of Sciences, Moscow, Russia 2 Pavlovsky Institu- te of Zoology and Parasitology, Academy of Sciences of Republic of Tajikistan, Dushanbe, Tajikistan 3 Institute of Molecular and Cellular Biology, Siberian Branch RAS, Novosibirsk, Russia 4 Novosibirsk State University, Novosibirsk, Russia 5 Cambridge Resource Centre for Comparative Genomics, Department of Veterinary Me- dicine, University of Cambridge, Cambridge, UK 6 Vavilov Institute of General Genetics, Russian Academy of Sciences, Moscow, Russia Corresponding author: Irina Bakloushinskaya ([email protected]) Academic editor: V. Lukhtanov | Received 1 March 2019 | Accepted 28 May 2019 | Published 20 June 2019 http://zoobank.org/4D72CDB3-20F3-4E24-96A9-72673C248856 Citation: Bakloushinskaya I, Lyapunova EA, Saidov AS, Romanenko SA, O’Brien PCM, Serdyukova NA, Ferguson- Smith MA, Matveevsky S, Bogdanov AS (2019) Rapid chromosomal evolution in enigmatic mammal with XX in both sexes, the Alay mole vole Ellobius alaicus Vorontsov et al., 1969 (Mammalia, Rodentia). Comparative Cytogenetics 13(2): 147–177. https://doi.org/10.3897/CompCytogen.v13i2.34224 Abstract Evolutionary history and taxonomic position for cryptic species may be clarified by using molecular and cy- togenetic methods. -

Tajikistan Overview
1 Tajikistan Overview The Republic of Tajikistan is a landlocked and mountainous country located in Central Asia. It shares political boundaries with four other countries; Kyrgyzstan to the north, China to the east, Afghanistan to the south, and Uzbekistan to the west. Tajikistan remains the poorest and most economically fragile of the former Soviet Republics. More than half of its population lives on less than US$2 a day. Tajikistan’s Flag The Tajikistan flag is made up of three stripes of which the middle white stripe is the largest. The white is used to symbolize purity and cotton as well as the snowy mountain peaks of Tajikistan. The red color is to represent the sun, the strength and unity of the nation along with victory. Green is the color of Islam and a representation of the gift of nature. The central crown surrounded by seven stars has two meanings. The crown is used to represent the people of Tajikistan, the seven stars is to show happiness and perfection. 1 Courtesy of CIA World Factbook Physical Geography Tajikistan constitutes an area of 143,100 sq km, over 90 percent of which is mountainous.2 The Trans- Alay Mountains lie in the northern portion of the country and are joined with the rugged Pamir Mountains by the Alay Valley. Tajikistan’s highest point at 7,495 meters Qullai Ismoili Somoni, was previously known as “Communism Peak”, and was the tallest mountain in the former USSR. While the lowest elevation in the country is roughly 300 meters, fifty percent of the country is at an elevation of over 3,000 meters.3 Large valleys allowing for expansive agriculture periodically punctuate the mountains. -

Download 3.72 MB
Report and Recommendation of the President to the Board of Directors Project Number: 39676 October 2007 Proposed Loan, Asian Development Fund Grants, and Technical Assistance Grant Kyrgyz Republic and Republic of Tajikistan: CAREC Regional Road Corridor Improvement Project CURRENCY EQUIVALENTS (as of 1 September 2007) TAJIKISTAN Currency Unit – somoni (TJS) TJS1.00 = $0.2905 $1.00 = TJS3.4418 KYRGYZ REPUBLIC Currency Unit – som (Som) Som1.00 = $0.0264 $1.00 = Som37.8100 ABBREVIATIONS AADT – annual average daily traffic ADB – Asian Development Bank CAR – Central Asian republic CAREC – Central Asia Regional Economic Cooperation CBA – cross-border agreement DEP – Ministry of Transport and Communications depot EA – executing agency EIRR – economic internal rate of return FSU – former Soviet Union GDP – gross domestic product HIV/AIDS – human immunodeficiency virus/acquired immunodeficiency syndrome ICB – international competitive bidding IEE – initial environmental examination KGZMOTC – Kyrgyz Ministry of Transport and Communications LAR – land acquisition and resettlement MOT – Ministry of Transport MOTC – Ministry of Transport and Communications NCB – national competitive bidding PBM – performance-based maintenance PIU – project implementation unit PMIS – project management information system PPMS – project performance management system PPTA – project preparatory technical assistance PRC – People’s Republic of China ROW – right-of-way RP – resettlement plan SIEE – summary initial environmental examination SRP – short resettlement plan TA – technical assistance TAJMOTC – Tajik Ministry of Transport and Communications NOTES In this report, "$" refers to US dollars Vice President L. Jin, Operations Group 1 Director General J. Miranda, Central and West Asia Department (CWRD) Director S. O’Sullivan, Infrastructure Division, CWRD Team leaders R. Ishenaliev, Transport Specialist, CWRD R. Sanda, Investment Specialist, CWRD Team members L. -

Asian Alpine E-News Issue No.37
ASIAN ALPINE E–NEWS Issue No.37, September 2018 CONTENTS Guidebook “Mountaineering and ski mountaineering areas of Kyrgyzstan” Page 2 ~ 48 Annotation New edition of the Guidebook “Mountaineering & ski mountaineering areas of Kyrgyzstan” (Fist edition was named “Mountaineering areas of Kyrgyzstan”) is continued publishing by Asian Alpine E-News. Part of the Guidebook already was published in issues No. 23&30 of the AAEN. The other articles (Chapters) will follow as far as ready. Part of articles are doing together with co-authors. 1 Guidebook “Mountaineering and ski mountaineering areas of Kyrgyzstan” Annotation New edition of the Guidebook “Mountaineering & ski mountaineering areas of Kyrgyzstan” (Fist edition was named “Mountaineering areas of Kyrgyzstan”) is continued publishing by Asian Alpine E-News. Part of the Guidebook already was published in issues No. 23&30 of the AAEN. The other articles (Chapters) will follow as far as ready. Part of articles are doing together with co-authors. The guide provides an overview and brief description (characteristics of areas, location, seasons, climate, climbing history, camps and bases, entrances and approaches, climbing opportunities) as well - developed mountaineering areas of Kyrgyzstan, and rarely visited and promising areas. There are described opportunities and recommendations on the organization, logistics of mountaineering expeditions, rescue operations, communications and formalities. The guide will be useful for those who are going to climb to Kyrgyzstan or choose new areas of the world for climbing. Author Vladimir Komissarov, PhD, IFMGA mountain guide, President of the Kyrgyz Mountain guides Association and the Kyrgyz Alpine Club, engaged in Mountaineering for more than 50 years. He has repeatedly visited and climbed all areas described by him. -

Sacred Sites of the Southern Kyrgyzstan: Nature, Manas, Islam
Aigine Cultural Research Center Sacred Sites of the Southern Kyrgyzstan: Nature, Manas, Islam Edited by Gulnara Aitpaeva Bishkek 2013 УДК 908 ББК 26.891 S 13 © Aigine Cultural Research Center, 2013 The research work and the publication was supported by The Christensen Fund, 260 Townsend St Suite 600 San Francisco, CA 94107 USA Author of the project, Gulnara Aitpaeva Working group, Aibek Samakov, Cholponai U-G, Gulnara Aitpaeva, Kyial Tajieva and Meerim Aitkeeva Design by Cholponai U-G Photographs by the team of the Aigine CRC, Aibek Samakov, Cholponai U-G, Guljan Kudabaeva and Gulnara Aitpaeva Sacred Site of the Southern Kyrgyzstan: Nature, Manas and Islam, Aigine Cultural S 13 Research Center. – B.: 2013, 240 p. ISBN 978-9967-27-146-3 This publication is a result of the participatory research conducted by the Aigine CRC, together with traditional practitioners and experts, in Batken, Jalalabad and Osh provinces for the last three years. The first part of the book contains oral history of natural and historical sacred sites, as well as, sacred sites related to Manas and Islam. The second part of the book contains life stories on interconnection of people, sacred sites, animals, plants and the stars. Printed and bound in Kyrgyzstan by St.art Ltd. S 1805080000-13 УДК 908 ISBN 978-9967-27-146-3 ББК 26.891 CONTENTS Note from the Editor 4 Acknowledgements 5 Introduction 6 Part 1 Sacred Sites in the South of Kyrgyzstan 11 Chapter 1 Natural Sacred Sites . 11 Sacred Lakes . 12 Sacred Caves . 18 Sacred Mountains . 21 Sacred Springs . 22 Sacred Rocks . -

Journal.Pone.0205646
Early pastoral economies along the Ancient Silk Road: Biomolecular evidence from the Alay Valley, Kyrgyzstan Taylor, William; Shnaider, Svetlana; Abdykanova, Aida; Fages, Antoine; Welker, Frido; Irmer, Franziska; Seguin-orlando, Andaine; Khan, Naveed; Douka, Katerina; Kolobova, Ksenia; Orlando, Ludovic; Krivoshapkin, Andrei; Boivin, Nicole; Hardy, Karen Published in: PLOS ONE DOI: 10.1371/journal.pone.0205646 Publication date: 2018 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Taylor, W., Shnaider, S., Abdykanova, A., Fages, A., Welker, F., Irmer, F., Seguin-orlando, A., Khan, N., Douka, K., Kolobova, K., Orlando, L., Krivoshapkin, A., Boivin, N., & Hardy, K. (Ed.) (2018). Early pastoral economies along the Ancient Silk Road: Biomolecular evidence from the Alay Valley, Kyrgyzstan. PLOS ONE, 13(10), [e0205646]. https://doi.org/10.1371/journal.pone.0205646 Download date: 29. sep.. 2021 RESEARCH ARTICLE Early pastoral economies along the Ancient Silk Road: Biomolecular evidence from the Alay Valley, Kyrgyzstan 1 2,3 4 5,6 William TaylorID *, Svetlana Shnaider , Aida Abdykanova , Antoine Fages , 7,8 1 5,6 6,9 Frido Welker , Franziska IrmerID , Andaine Seguin-Orlando , Naveed KhanID , 1 2 5,6 2,10 Katerina Douka , Ksenia Kolobova , Ludovic Orlando , Andrei KrivoshapkinID , Nicole Boivin1 a1111111111 1 Department of Archaeology, Max Planck Institute for the Science of Human History, Jena, Germany, 2 Institute of Archaeology and Ethnography, Siberian Branch, -

Epidemic Cystic and Alveolar Echinococcosis in Kyrgyzstan: an Analysis of National Surveillance Data
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2020 Epidemic cystic and alveolar echinococcosis in Kyrgyzstan: an analysis of national surveillance data Paternoster, Giulia ; Boo, Gianluca ; Wang, Craig ; Minbaeva, Gulnara ; Usubalieva, Jumagul ; Raimkulov, Kursanbek Mamasalievich ; Zhoroev, Abdykadyr ; Abdykerimov, Kubanychbek Kudaibergenovich ; Kronenberg, Philipp Andreas ; Müllhaupt, Beat ; Furrer, Reinhard ; Deplazes, Peter ; Torgerson, Paul R Abstract: Background Human cystic and alveolar echinococcosis are among the priority neglected zoonotic diseases for which WHO advocates control. The incidence of both cystic echinococcosis and alveolar echinococcosis has increased substantially in the past 30 years in Kyrgyzstan. Given the scarcity of adequate data on the local geographical variation of these focal diseases, we aimed to investigate within- country incidence and geographical variation of cystic echinococcosis and alveolar echinococcosis at a high spatial resolution in Kyrgyzstan. Methods We mapped all confirmed surgical cases of cystic echinococcosis and alveolar echinococcosis reported through the national echinococcosis surveillance system in Kyrgyzs- tan between Jan 1, 2014, and Dec 31, 2016, from nine regional databases. We then estimated crude surgical incidence, standardised incidence, and standardised incidence ratios (SIRs) of primary cases (ie, excluding relapses) based on age and sex at country, region, district, and local community levels. Finally, we tested the SIRs for global and local spatial autocorrelation to identify disease hotspots at the local community level. All incidence estimates were calculated per 100 000 population and averaged across the 3-year study period to obtain annual estimates. Findings The surveillance system reported 2359 primary surgical cases of cystic echinococcosis and 546 primary surgical cases of alveolar echinococcosis. -

Taxonomic Corrections and New Records in Vascular Plants of Kyrgyzstan, 4
Memoranda Soc. Soc. Fauna Fauna Flora Flora Fennica Fennica 91, 91: 2015 67–83. • Lazkov 2015 & Sennikov 67 Taxonomic corrections and new records in vascular plants of Kyrgyzstan, 4 Georgy A. Lazkov & Alexander N. Sennikov* Lazkov, G.A., Laboratory of Flora, Institute of Biology and Soil Science, Kyrgyz Academy of Sciences, 720071 Bishkek, Kyrgyzstan. E-mail: [email protected] Sennikov, A.N., Botanical Museum, Finnish Museum of Natural History, P.O. Box 7, 00014 University of Helsinki, Finland; & Herbarium, Komarov Botanical Institute of Russian Academy of Sciences, Prof. Popov str. 2, 197376 St Petersburg, Russia. E-mail: alexander.sennikov@ helsinki.fi (*Author for correspondence) A new series of notes on distribution, taxonomy, morphology and nomenclature of some vascular plants in Kyrgyzstan is presented. Carex subphysodes Popov ex V.Krecz., Astragalus sogdianus Bunge, Oxytropis ferganensis Vass. and Iris maracandica (Vved.) Wendelbo (all native), and also Delphinium orientalis J.Gay (alien) are reported as new to Kyrgyzstan. Sedum tetramerum Trautv. is new to Northern Tian-Shan, and Scirpoides holoschoenus (L.) Soják is new to Chatkal Range and Western Tian-Shan within Kyrgyzstan. The distribution area of Torilis arvensis (Huds.) Link is revised and expanded, and the distribution of Eremurus zoae Vved. (endemic to Kyrgyzstan) is verified and mapped. New names and combinations, Betonica sect. Foliosae (Krestovsk. & Laz- kov) Lazkov, Eriophyton anomalum (Juz.) Lazkov & Sennikov, Kudrjaschevia sect. Jacubianae Lazkov, Lagochilus sect. Chlainanthus (Briq.) Lazkov, Leonurus sect. Panzerioidei (Krestovsk.) Lazkov, Phlomoides sect. Pseuderemostachys (Popov) Lazkov, and Scutellaria sect. Ramosissi- mae Lazkov, are provided as a result of the forthcoming monographic revision of Lamiaceae. Two hybrids are described in Eremurus, E.