Genital Prolapse Surgery: What Options Do We Have in the Age of Mesh Issues?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tension Free Femoral Hernia Repair with Plug Milivoje Vuković1, Nebojša Moljević1, Siniša Crnogorac2
Journal of Acute Disease (2013)40-43 40 Contents lists available at ScienceDirect Journal of Acute Disease journal homepage: www.jadweb.org Document heading doi: 10.1016/S2221-6189(13)60093-1 Tension free femoral hernia repair with plug Milivoje Vuković1, Nebojša Moljević1, Siniša Crnogorac2 1Clinical Center of Vojvodina, Clinic for Abdominal, Endocrine and Transplantation Surgery, Novi Sad, Serbia 2Clinical Center of Vojvodina, Emergency Center, Novi Sad, Serbia ARTICLE INFO ABSTRACT Article history: Objective: To investigate the conventional technique involves treatment of femoral hernia an Received 10 January 2012 approximation inguinal ligament to pectinealMethod: ligament. In technique which uses mesh closure for Received in revised form 15 March 2012 femoral canal without tissue tension. A prospective study from January 01. 2007-May Accepted 15 May 2012 30. 2009. We analyzed 1 042 patients with inguinal hernia, of which there were 83 patients with 86 Available online 20 November 2012 Result: femoral hernia. Femoral hernias were present in 7.96% of cases. Males were 13 (15.66%) and 70 women (84.34%). The gender distribution of men: women is 1:5.38. Urgent underwent 69 Keywords: (83%), and the 14 election (17%) patients. Average age was 63 years, the youngest patient was a Femoral hernia 24 and the oldest 86 years. Ratio of right: left hernias was 3.4:1. With bilateral femoral hernias % ( %) Mesh+plug Conclusions:was 3.61 of cases. In 7 patients 8.43 underwent femoral hernia repair with 9 Prolene plug. Hernioplasty The technique of closing the femoral canal with plug a simple. The plug is made from monofilament material and is easily formed. -

Anatomical Study on the Psoas Minor Muscle in Human Fetuses
Int. J. Morphol., 30(1):136-139, 2012. Anatomical Study on the Psoas Minor Muscle in Human Fetuses Estudio Anatómico del Músculo Psoas Menor en Fetos Humanos *Danilo Ribeiro Guerra; **Francisco Prado Reis; ***Afrânio de Andrade Bastos; ****Ciro José Brito; *****Roberto Jerônimo dos Santos Silva & *,**José Aderval Aragão GUERRA, D. R.; REIS, F. P.; BASTOS, A. A.; BRITO, C. J.; SILVA, R. J. S. & ARAGÃO, J. A. Anatomical study on the psoas minor muscle in human fetuses. Int. J. Morphol., 30(1):136-139, 2012. SUMMARY: The anatomy of the psoas minor muscle in human beings has frequently been correlated with ethnic and racial characteristics. The present study had the aim of investigating the anatomy of the psoas minor, by observing its occurrence, distal insertion points, relationship with the psoas major muscle and the relationship between its tendon and muscle portions. Twenty-two human fetuses were used (eleven of each gender), fixed in 10% formol solution that had been perfused through the umbilical artery. The psoas minor muscle was found in eight male fetuses: seven bilaterally and one unilaterally, in the right hemicorpus. Five female fetuses presented the psoas minor muscle: three bilaterally and two unilaterally, one in the right and one in the left hemicorpus. The muscle was independent, inconstant, with unilateral or bilateral presence, with distal insertions at different anatomical points, and its tendon portion was always longer than the belly of the muscle. KEY WORDS: Psoas Muscles; Muscle, Skeletal; Anatomy; Gender Identity. INTRODUCTION When the psoas minor muscle is present in humans, The aim of the present study was to investigate the it is located in the posterior wall of the abdomen, laterally to anatomy of the psoas minor muscle in human fetuses: the lumbar spine and in close contact and anteriorly to the establishing the frequency of its occurrence according to sex; belly of the psoas major muscle (Van Dyke et al., 1987; ascertaining the distal insertion points; analyzing the possible Domingo, Aguilar et al., 2004; Leão et al., 2007). -

Anterior Abdominal Wall
Abdominal wall Borders of the Abdomen • Abdomen is the region of the trunk that lies between the diaphragm above and the inlet of the pelvis below • Borders Superior: Costal cartilages 7-12. Xiphoid process: • Inferior: Pubic bone and iliac crest: Level of L4. • Umbilicus: Level of IV disc L3-L4 Abdominal Quadrants Formed by two intersecting lines: Vertical & Horizontal Intersect at umbilicus. Quadrants: Upper left. Upper right. Lower left. Lower right Abdominal Regions Divided into 9 regions by two pairs of planes: 1- Vertical Planes: -Left and right lateral planes - Midclavicular planes -passes through the midpoint between the ant.sup.iliac spine and symphysis pupis 2- Horizontal Planes: -Subcostal plane - at level of L3 vertebra -Joins the lower end of costal cartilage on each side -Intertubercular plane: -- At the level of L5 vertebra - Through tubercles of iliac crests. Abdominal wall divided into:- Anterior abdominal wall Posterior abdominal wall What are the Layers of Anterior Skin Abdominal Wall Superficial Fascia - Above the umbilicus one layer - Below the umbilicus two layers . Camper's fascia - fatty superficial layer. Scarp's fascia - deep membranous layer. Deep fascia : . Thin layer of C.T covering the muscle may absent Muscular layer . External oblique muscle . Internal oblique muscle . Transverse abdominal muscle . Rectus abdominis Transversalis fascia Extraperitoneal fascia Parietal Peritoneum Superficial Fascia . Camper's fascia - fatty layer= dartos muscle in male . Scarpa's fascia - membranous layer. Attachment of scarpa’s fascia= membranous fascia INF: Fascia lata Sides: Pubic arch Post: Perineal body - Membranous layer in scrotum referred to as colle’s fascia - Rupture of penile urethra lead to extravasations of urine into(scrotum, perineum, penis &abdomen) Muscles . -

1 Anatomy of the Abdominal Wall 1
Chapter 1 Anatomy of the Abdominal Wall 1 Orhan E. Arslan 1.1 Introduction The abdominal wall encompasses an area of the body boundedsuperiorlybythexiphoidprocessandcostal arch, and inferiorly by the inguinal ligament, pubic bones and the iliac crest. Epigastrium Visualization, palpation, percussion, and ausculta- Right Left tion of the anterolateral abdominal wall may reveal ab- hypochondriac hypochondriac normalities associated with abdominal organs, such as Transpyloric T12 Plane the liver, spleen, stomach, abdominal aorta, pancreas L1 and appendix, as well as thoracic and pelvic organs. L2 Right L3 Left Visible or palpable deformities such as swelling and Subcostal Lumbar (Lateral) Lumbar (Lateral) scars, pain and tenderness may reflect disease process- Plane L4 L5 es in the abdominal cavity or elsewhere. Pleural irrita- Intertuber- Left tion as a result of pleurisy or dislocation of the ribs may cular Iliac (inguinal) Plane result in pain that radiates to the anterior abdomen. Hypogastrium Pain from a diseased abdominal organ may refer to the Right Umbilical Iliac (inguinal) Region anterolateral abdomen and other parts of the body, e.g., cholecystitis produces pain in the shoulder area as well as the right hypochondriac region. The abdominal wall Fig. 1.1. Various regions of the anterior abdominal wall should be suspected as the source of the pain in indi- viduals who exhibit chronic and unremitting pain with minimal or no relationship to gastrointestinal func- the lower border of the first lumbar vertebra. The sub- tion, but which shows variation with changes of pos- costal plane that passes across the costal margins and ture [1]. This is also true when the anterior abdominal the upper border of the third lumbar vertebra may be wall tenderness is unchanged or exacerbated upon con- used instead of the transpyloric plane. -

Joint Report on Terminology for Surgical Procedures to Treat Pelvic
AUGS-IUGA JOINT PUBLICATION Joint Report on Terminology for Surgical Procedures to Treat Pelvic Organ Prolapse Developed by the Joint Writing Group of the American Urogynecologic Society and the International Urogynecological Association. Individual contributors are noted in the acknowledgment section. 03/02/2020 on BhDMf5ePHKav1zEoum1tQfN4a+kJLhEZgbsIHo4XMi0hCywCX1AWnYQp/IlQrHD3JfJeJsayAVVC6IBQr6djgLHr3m8XRMZF6k61FXizrL9aj3Mm1iL7ZA== by https://journals.lww.com/jpelvicsurgery from Downloaded meaningful data about specific procedures, standardized and Downloaded Abstract: Surgeries for pelvic organ prolapse (POP) are common, but widely accepted terminology must be adopted. Each term for a standardization of surgical terms is needed to improve the quality of in- given procedure must indicate to researchers, clinicians, and from vestigation and clinical care around these procedures. The American learners a specific and reliable minimal set of steps. The aim of https://journals.lww.com/jpelvicsurgery Urogynecologic Society and the International Urogynecologic Associ- this document is to propose a standardized terminology to de- ation convened a joint writing group consisting of 5 designees from scribe common surgeries for POP. each society to standardize terminology around common surgical terms in POP repair including the following: sacrocolpopexy (including sacral colpoperineopexy), sacrocervicopexy, uterosacral ligament suspension, sacrospinous ligament fixation, iliococcygeus fixation, uterine preserva- tion prolapse procedures or hysteropexy -

Morbidity and Mortality Hernia Repair
MorbidityMorbidity andand MortalityMortality HerniaHernia RepairRepair KingsKings CountyCounty HospitalHospital AugustAugust 18,18, 20062006 JoelleJoelle PierrePierre CaseCase PresentationPresentation xxxx y/oy/o malemale withwith h/oh/o ESRDESRD presentedpresented toto KingsKings CountyCounty HospitalHospital forfor repairrepair ofof aa rightright inguinalinguinal herniahernia LabsLabs prepre--opop :: HctHct 43.1,43.1, PTPT :11.8,:11.8, PTTPTT 31.631.6 HemodialysisHemodialysis:: 11 dayday prepre--opop PtPt underwentunderwent anan uneventfuluneventful rightright inguinalinguinal herniahernia repairrepair withwith patchpatch andand plugplug systemsystem andand waswas dischargeddischarged home.home. CourseCourse POD#1POD#1 :: PtPt receivedreceived hemodialysishemodialysis withwith 3,000U3,000U ofof heparinheparin POD#POD# 44 :: PtPt returnedreturned toto thethe ERER complainingcomplaining ofof swellingswelling toto thethe rightright inguinalinguinal region.region. HctHct :: 3535 PTPT 11.4,11.4, PTTPTT 22.022.0 PtPt hadhad anan AXRAXR andand CTCT ScanScan ofof thethe AbdomenAbdomen CourseCourse continuedcontinued PtPt waswas admittedadmitted forfor observationobservation andand IVIV atbxatbx HematomaHematoma waswas stablestable andand thethe swellingswelling decreaseddecreased HctHct stabilizedstabilized atat 3030 PtPt waswas dischargeddischarged homehome onon POPO atbxatbx ComplicationsComplications ofof InguinalInguinal HerniaHernia RepairRepair AugustAugust 18,18, 20042004 InguinalInguinal herniahernia repair:repair: herniarrophyherniarrophy -

2. Abdominal Wall and Hernias
BWH 2015 GENERAL SURGERY RESIDENCY PROCEDURAL ANATOMY COURSE 2. ABDOMINAL WALL AND HERNIAS Contents LAB OBJECTIVES ............................................................................................................................................... 2 Knowledge objectives .................................................................................................................................. 2 Skills objectives ............................................................................................................................................ 2 Preparation for lab .......................................................................................................................................... 2 1.1 ORGANIZATION OF THE ABDOMINAL WALL ............................................................................................ 4 Organization of the trunk wall .................................................................................................................... 4 Superficial layers of the trunk wall ............................................................................................................. 5 Musculoskeletal layer of the anterolateral abdominal wall ...................................................................... 7 T3/Deep fascia surrounding the musculoskeletal layer of the abdominal wall ..................................... 11 Deeper layers of the trunk wall ............................................................................................................... -

Inguinal Canal Inguinal Ligament
Inguinal canal Inguinal Ligament: An in ward folding of external oblique aponeurosis which extending between anterior superior iliac spine and pubic tubercle. The lateral part of inguinal ligament gives origin to the internal oblique and transversus abdominis muscles. Its inferior rounded border is attached to the deep fascia of the thigh "fascia lata” Lacunar ligament: It arise from medial end of inguinal ligament. It extend backward & upward to the superior ramus of pubis. Its free crescentic edge is sharp & forms medial margin of femoral ring. Pectineal ligament :Its attachment of lacunar ligament to periosteum of pectineal line. Superficial inguinal ring: A triangular shape defect in external oblique aponeurosis above & medial to pubic tubercle. Through it pass spermatic cord (or round ligament of uterus) carrying with it a covering called external spermatic fascia (or external covering of round ligament of uterus) from margin of the ring. Deep inguinal ring: It lying 1.3 cm above inguinal ligament midway. Its an oval opening in transversalis fascia. From deep ring margin the spermatic cord gain a covering called internal spermatic fascia. Inguinal canal: It is a intramuscular slit lying above medial half of the inguinal ligament, it is 4 cm long . It starts at deep inguinal ring &ends at superficial inguinal ring. It transmit spermatic cord in male & round ligament of uterus in female. Inguinal Canal Walls: Anterior wall: EO aponeurosis & helped laterally by IO.**** Floor: The inguinal ligament reinforced medially by lacunar ligament. Roof: IO & TA muscles laterally conjoint tendon medially Posterior wall: medially conjoint tendon, while laterally it’s the weak transversalis fascia Contents of Inguinal canal: In males: spermatic cord In females: it’s a smaller canal, permit passage of round ligament of uterus In both sexes: it also transmits ilio inguinal nerve. -

Joint Report on Terminology for Surgical Procedures to Treat Pelvic Organ Prolapse
International Urogynecology Journal https://doi.org/10.1007/s00192-020-04236-1 AUGS-IUGA JOINT PUBLICATION Joint report on terminology for surgical procedures to treat pelvic organ prolapse Developed by the Joint Writing Group of the American Urogynecologic Society and the International Urogynecological Association # American Urogynecologic Society and International Urogynecological Association 2020 Abstract Surgeries for pelvic organ prolapse (POP) are common, but standardization of surgical terms is needed to improve the quality of investigation and clinical care around these procedures. The American Urogynecologic Society and the International Urogynecologic Association convened a joint writing group consisting of 5 designees from each society to standardize terminology around common surgical terms in POP repair including the following: sacrocolpopexy (including sacral colpoperineopexy), sacrocervicopexy, uterosacral ligament suspension, sacrospinous ligament fixation, iliococcygeus fixation, uterine preservation prolapse procedures or hysteropexy (including sacrohysteropexy, uterosacral hysteropexy, sacrospinous hysteropexy, anterior abdominal wall hysteropexy, Manchester procedure), anterior prolapse procedures (including anterior vaginal repair, anterior vaginal repair with graft, and paravaginal repair), posterior prolapse procedures (including posterior vaginal repair, posterior vaginal repair with graft, levator plication, and perineal repair), and obliterative prolapse repairs (including colpocleisis with hysterectomy, colpocleisis -
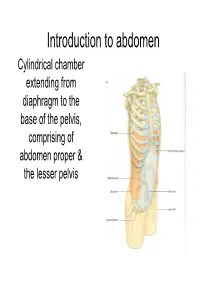
Introduction to Abdoman
Introduction to abdomen Cylindrical chamber extending from A diaphragm to the base of the pelvis, comprising of abdomen proper & the lesser pelvis -+------- Lower limb • Abdomen proper & lesser pelvis communicate with each other at the plane of inlet into lesser pelvis (upper border of pubic symphysis,pubic crests, arcuate line of innominate bones,sacral promontary) pelvis Pelvic inlet Inguinal ligament • Contents of Abdomen proper:- Most of the digestive tube, Liver, pancreas, spleen, kidneys, ureters (in part), supra renal gland & various blood &lymph vessels lymph nodes &nerves • Contents of lesser pelvis:- Terminal parts of ureters, urinary bladders, the sigmoid colon, rectum some coils of ileum, internal genitalia, blood & lymph vesels, lymph nodes & nerves Functions • Houses & protects major viscera Rib cage Assists in breathing of Relaxation of diaphragm diaphragm Relaxation of abdominal muscles Contraction of abdominal muscles Expiration Inspiration Changes in the intra abdominal pressure Laryngeai cav'ity c·1os 1erd Cr0ntrac ion of abdominal wal I ncrease in intra- abdominal pressure _..l,....._ Mictu ·ition Child birth Defecation Component parts ony Frame,vork of Abdomen • Wall- Skeletal elements Muscles • Muscles:- Superficial fascia • Anteriorly a Fatty layer Membranous layer (Camper's) (Scarpa's) segmented Transversalis fascia muscle Rectus [=E<trapedtoceru..; abdominis Parietal peritoneum Visceral peritoneum • Anterolateraly External oblique, internal oblique & trasversus abdominis ~.34 Transverse section showing the layers of the abdominal wa ll. Po~ t ei·i 01· .--\. lJcl 0111in a] \,-a]] • Posteriorly- Quadratus lumborum, psoas major & iliacus Abdominal regions Subcostal plane Midclavicular planes ,7 I I Left lowe ( I. _ quadr;mt Transumbilical plane Median plane Transtubercular plane Regions on anterior abdominal wall vertic~I plane Left ve rtical p,lane ' Hypochondriac Hypochondriac L / ' / ' ,/ '' / ' ✓ ' Subcostal plane ,-.;;'-~_,,:;.....,,,___ --i--------------~+-----'_ ......;;_..,,___. -

Original Article
ORIGINAL ARTICLE MORPHOLOGY OF PSOAS MINOR MUSCLE - REVIEWED Sonali Agichani1, Yogesh Sontakke2, S.S. Joshi3, S.D. Joshi4 HOW TO CITE THIS ARTICLE: Sonali Agichani, Yogesh Sontakke, S. S. Joshi, S. D. Joshi. “Morphology of psoas minor muscle - reviewed”. Journal of Evolution of Medical and Dental Sciences 2013; Vol2, Issue 31, August 5; Page: 5867-5874. ABSTRACT: Psoas minor (PM) muscle belongs to the category of vestigial muscles. It is large in size in all those quadrupeds that brachiate and leap or run at very fast speed. None of these functions being required in bipedal, plantigrade man the muscle has receded during evolution; hence it is present only in 40-60% population. Apart from racial variations, a large number of morphological variations of this muscle have been described in the literature. The present study has been conducted in 20 cadavers. Psoas minor muscle was present bilaterally in 35% cases and unilaterally in 5% cases; overall incidence being 40%. Average length of fleshy belly was 7.85 cm that of tendon was 13.13 cm. Average maximum width of fleshy belly was 1.93 cm, and that of the tendon was 0.77cm. In most of the cases, muscle originated from the sides of bodies of T12 & L1 vertebrae & their intervening intervertebral disc. In few of them, origin extended to the sub diaphragmatic fascia & the medial arcuate ligament (Fig.1a). Tendon of PM flattened out at insertion on iliopectineal line & blended with iliopsoas fascia (Fig.2a, 3a). The expansion of tendon into this fascia might be serving some special functions, hitherto fore unappreciated. -

New Procedures for Uterine Prolapse
Best Practice & Research Clinical Obstetrics and Gynaecology 27 (2013) 363–379 Contents lists available at SciVerse ScienceDirect Best Practice & Research Clinical Obstetrics and Gynaecology journal homepage: www.elsevier.com/locate/bpobgyn 5 New procedures for uterine prolapse Azar Khunda, MRCOG, Subspecialty Fellow in Urogynaecology *, Arvind Vashisht, MA MD, MRCOG, Consultant Urogynaecologist, Alfred Cutner, MD, FRCOG, Consultant Gynaecologist UCLH Urogynaecology and Pelvic Floor Unit, University College Hospital, 235 Euston Road, London NW1 2BU, UK Keywords: Traditionally, vaginal hysterectomy and Manchester repair were sacrospinous hysteropexy the surgical approaches to treating uterine prolapse; however, sacrohysteropexy both are associated with a relatively high subsequent vaginal vault uterine suspension recurrence. Laparoscopic uterine suspension is a new way of prolapse maintaining uterine support. Many women are keen to keep their uterus for a variety of reasons, including maintaining reproductive capability and the belief that the uterus, cervix, or both, may play a part of their gender identity. Non-removal of the uterus may retain functional (e.g. bowel, bladder and sexual) benefits. There- fore, the concept of uterine preservation for pelvic-organ prolapse has been of interest to pelvic-floor surgeons for many decades. In this review, we provide an overview of the available evidence on treating uterine prolapse surgically. We describe techniques to support the vault during hysterectomy, and examine the evidence for uterine-sparing