Joint Report on Terminology for Surgical Procedures to Treat Pelvic Organ Prolapse
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tension Free Femoral Hernia Repair with Plug Milivoje Vuković1, Nebojša Moljević1, Siniša Crnogorac2
Journal of Acute Disease (2013)40-43 40 Contents lists available at ScienceDirect Journal of Acute Disease journal homepage: www.jadweb.org Document heading doi: 10.1016/S2221-6189(13)60093-1 Tension free femoral hernia repair with plug Milivoje Vuković1, Nebojša Moljević1, Siniša Crnogorac2 1Clinical Center of Vojvodina, Clinic for Abdominal, Endocrine and Transplantation Surgery, Novi Sad, Serbia 2Clinical Center of Vojvodina, Emergency Center, Novi Sad, Serbia ARTICLE INFO ABSTRACT Article history: Objective: To investigate the conventional technique involves treatment of femoral hernia an Received 10 January 2012 approximation inguinal ligament to pectinealMethod: ligament. In technique which uses mesh closure for Received in revised form 15 March 2012 femoral canal without tissue tension. A prospective study from January 01. 2007-May Accepted 15 May 2012 30. 2009. We analyzed 1 042 patients with inguinal hernia, of which there were 83 patients with 86 Available online 20 November 2012 Result: femoral hernia. Femoral hernias were present in 7.96% of cases. Males were 13 (15.66%) and 70 women (84.34%). The gender distribution of men: women is 1:5.38. Urgent underwent 69 Keywords: (83%), and the 14 election (17%) patients. Average age was 63 years, the youngest patient was a Femoral hernia 24 and the oldest 86 years. Ratio of right: left hernias was 3.4:1. With bilateral femoral hernias % ( %) Mesh+plug Conclusions:was 3.61 of cases. In 7 patients 8.43 underwent femoral hernia repair with 9 Prolene plug. Hernioplasty The technique of closing the femoral canal with plug a simple. The plug is made from monofilament material and is easily formed. -
Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010
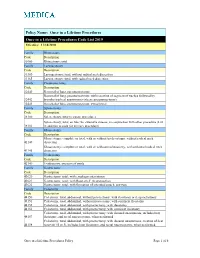
Policy Name: Once in a Lifetime Procedures Once in a Lifetime Procedures Code List 2019 Effective: 11/14/2010 Family Rhinectomy Code Description 30160 Rhinectomy; total Family Laryngectomy Code Description 31360 Laryngectomy; total, without radical neck dissection 31365 Laryngectomy; total, with radical neck dissection Family Pneumonectomy Code Description 32440 Removal of lung, pneumonectomy; Removal of lung, pneumonectomy; with resection of segment of trachea followed by 32442 broncho-tracheal anastomosis (sleeve pneumonectomy) 32445 Removal of lung, pneumonectomy; extrapleural Family Splenectomy Code Description 38100 Splenectomy; total (separate procedure) Splenectomy; total, en bloc for extensive disease, in conjunction with other procedure (List 38102 in addition to code for primary procedure) Family Glossectomy Code Description Glossectomy; complete or total, with or without tracheostomy, without radical neck 41140 dissection Glossectomy; complete or total, with or without tracheostomy, with unilateral radical neck 41145 dissection Family Uvulectomy Code Description 42140 Uvulectomy, excision of uvula Family Gastrectomy Code Description 43620 Gastrectomy, total; with esophagoenterostomy 43621 Gastrectomy, total; with Roux-en-Y reconstruction 43622 Gastrectomy, total; with formation of intestinal pouch, any type Family Colectomy Code Description 44150 Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy 44151 Colectomy, total, abdominal, without proctectomy; with continent ileostomy 44155 Colectomy, -

Overview of Surgical Techniques in Gender-Affirming Genital Surgery
208 Review Article Overview of surgical techniques in gender-affirming genital surgery Mang L. Chen1, Polina Reyblat2, Melissa M. Poh2, Amanda C. Chi2 1GU Recon, Los Angeles, CA, USA; 2Southern California Permanente Medical Group, Los Angeles, CA, USA Contributions: (I) Conception and design: ML Chen, AC Chi; (II) Administrative support: None; (III) Provision of study materials or patients: All authors; (IV) Collection and assembly of data: None; (V) Data analysis and interpretation: None; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Amanda C. Chi. 6041 Cadillac Ave, Los Angeles, CA 90034, USA. Email: [email protected]. Abstract: Gender related genitourinary surgeries are vitally important in the management of gender dysphoria. Vaginoplasty, metoidioplasty, phalloplasty and their associated surgeries help patients achieve their main goal of aligning their body and mind. These surgeries warrant careful adherence to reconstructive surgical principles as many patients can require corrective surgeries from complications that arise. Peri- operative assessment, the surgical techniques employed for vaginoplasty, phalloplasty, metoidioplasty, and their associated procedures are described. The general reconstructive principles for managing complications including urethroplasty to correct urethral bulging, vaginl stenosis, clitoroplasty and labiaplasty after primary vaginoplasty, and urethroplasty for strictures and fistulas, neophallus and neoscrotal reconstruction after phalloplasty are outlined as well. Keywords: Transgender; vaginoplasty; phalloplasty; metoidioplasty Submitted May 30, 2019. Accepted for publication Jun 20, 2019. doi: 10.21037/tau.2019.06.19 View this article at: http://dx.doi.org/10.21037/tau.2019.06.19 Introduction the rectum and the lower urinary tract, formation of perineogenital complex for patients who desire a functional The rise in social awareness of gender dysphoria has led vaginal canal, labiaplasty, and clitoroplasty. -

Cervical Cancer
Cervical Cancer Ritu Salani, M.D., M.B.A. Assistant Professor, Dept. of Obstetrics & Gynecology Division of Gynecologic Oncology The Ohio State University Estimated gynecologic cancer cases United States 2010 Jemal, A. et al. CA Cancer J Clin 2010; 60:277-300 1 Estimated gynecologic cancer deaths United States 2010 Jemal, A. et al. CA Cancer J Clin 2010; 60:277-300 Decreasing Trends of Cervical Cancer Incidence in the U.S. • With the advent of the Pap smear, the incidence of cervical cancer has dramatically declined. • The curve has been stable for the past decade because we are not reaching the unscreened population. Reprinted by permission of the American Cancer Society, Inc. 2 Cancer incidence worldwide GLOBOCAN 2008 Cervical Cancer New cases Deaths United States 12,200 4,210 Developing nations 530,000 275,000 • 85% of cases occur in developing nations ¹Jemal, CA Cancer J Clin 2010 GLOBOCAN 2008 3 Cervical Cancer • Histology – Squamous cell carcinoma (80%) – Adenocarcinoma (15%) – Adenosquamous carcinoma (3 to 5%) – Neuroendocrine or small cell carcinoma (rare) Human Papillomavirus (HPV) • Etiologic agent of cervical cancer • HPV DNA sequences detected is more than 99% of invasive cervical carcinomas • High risk types: 16, 18, 45, and 56 • Intermediate types: 31, 33, 35, 39, 51, 52, 55, 58, 59, 66, 68 HPV 16 accounts for ~80% of cases HPV 18 accounts for 25% of cases Walboomers JM, Jacobs MV, Manos MM, et al. J Pathol 1999;189(1):12-9. 4 Viral persistence and Precancerous progression Normal lesion cervix Regression/ clearance Invasive cancer Risk factors • Early age of sexual activity • Cigggarette smoking • Infection by other microbial agents • Immunosuppression – Transplant medications – HIV infection • Oral contraceptive use • Dietary factors – Deficiencies in vitamin A and beta carotene 5 Multi-Stage Cervical Carcinogenesis Rosenthal AN, Ryan A, Al-Jehani RM, et al. -

Anatomical Study on the Psoas Minor Muscle in Human Fetuses
Int. J. Morphol., 30(1):136-139, 2012. Anatomical Study on the Psoas Minor Muscle in Human Fetuses Estudio Anatómico del Músculo Psoas Menor en Fetos Humanos *Danilo Ribeiro Guerra; **Francisco Prado Reis; ***Afrânio de Andrade Bastos; ****Ciro José Brito; *****Roberto Jerônimo dos Santos Silva & *,**José Aderval Aragão GUERRA, D. R.; REIS, F. P.; BASTOS, A. A.; BRITO, C. J.; SILVA, R. J. S. & ARAGÃO, J. A. Anatomical study on the psoas minor muscle in human fetuses. Int. J. Morphol., 30(1):136-139, 2012. SUMMARY: The anatomy of the psoas minor muscle in human beings has frequently been correlated with ethnic and racial characteristics. The present study had the aim of investigating the anatomy of the psoas minor, by observing its occurrence, distal insertion points, relationship with the psoas major muscle and the relationship between its tendon and muscle portions. Twenty-two human fetuses were used (eleven of each gender), fixed in 10% formol solution that had been perfused through the umbilical artery. The psoas minor muscle was found in eight male fetuses: seven bilaterally and one unilaterally, in the right hemicorpus. Five female fetuses presented the psoas minor muscle: three bilaterally and two unilaterally, one in the right and one in the left hemicorpus. The muscle was independent, inconstant, with unilateral or bilateral presence, with distal insertions at different anatomical points, and its tendon portion was always longer than the belly of the muscle. KEY WORDS: Psoas Muscles; Muscle, Skeletal; Anatomy; Gender Identity. INTRODUCTION When the psoas minor muscle is present in humans, The aim of the present study was to investigate the it is located in the posterior wall of the abdomen, laterally to anatomy of the psoas minor muscle in human fetuses: the lumbar spine and in close contact and anteriorly to the establishing the frequency of its occurrence according to sex; belly of the psoas major muscle (Van Dyke et al., 1987; ascertaining the distal insertion points; analyzing the possible Domingo, Aguilar et al., 2004; Leão et al., 2007). -

Gender Dysphoria Treatment – Community Plan Medical Policy
UnitedHealthcare® Community Plan Medical Policy Gender Dysphoria Treatment Policy Number: CS145.I Effective Date: March 1, 2021 Instructions for Use Table of Contents Page Related Community Plan Policies Application ..................................................................................... 1 • Blepharoplasty, Blepharoptosis, and Brow Ptosis Coverage Rationale ....................................................................... 1 Repair Definitions ...................................................................................... 3 • Botulinum Toxins A and B Applicable Codes .......................................................................... 4 • Cosmetic and Reconstructive Procedures Description of Services ................................................................. 8 • Gonadotropin Releasing Hormone Analogs Benefit Considerations .................................................................. 9 Clinical Evidence ........................................................................... 9 • Panniculectomy and Body Contouring Procedures U.S. Food and Drug Administration ........................................... 14 • Rhinoplasty and Other Nasal Surgeries References ................................................................................... 14 • Speech Language Pathology Services Policy History/Revision Information ........................................... 16 Commercial Policy Instructions for Use ..................................................................... 16 • Gender Dysphoria Treatment -

Diagnosing and Managing Vulvar Disease
Diagnosing and Managing Vulvar Disease John J. Willems, M.D. FRCSC, FACOG Chairman, Department of Obstetrics & Gynecology Scripps Clinic La Jolla, California Objectives: IdentifyIdentify thethe majormajor formsforms ofof vulvarvulvar pathologypathology DescribeDescribe thethe appropriateappropriate setupsetup forfor vulvarvulvar biopsybiopsy DescribeDescribe thethe mostmost appropriateappropriate managementmanagement forfor commonlycommonly seenseen vulvarvulvar conditionsconditions Faculty Disclosure Unlabeled Product Company Nature of Affiliation Usage Warner Chilcott Speakers Bureau None ClassificationClassification ofof VulvarVulvar DiseaseDisease byby ClinicalClinical CharacteristicCharacteristic • Red lesions • White lesions • Dark lesions •Ulcers • Small tumors • Large tumors RedRed LesionsLesions • Candida •Tinea • Reactive vulvitis • Seborrheic dermatitis • Psoriasis • Vulvar vestibulitis • Paget’s disease Candidal vulvitis Superficial grayish-white film is often present Thick film of candida gives pseudo-ulcerative appearance. Acute vulvitis from coital trauma Contact irritation from synthetic fabrics Nomenclature SubtypesSubtypes ofof VulvodyniaVulvodynia:: VulvarVulvar VestibulitisVestibulitis SyndromeSyndrome (VVS)(VVS) alsoalso knownknown asas:: • Vestibulodynia • localized vulvar dysesthesia DysestheticDysesthetic VulvodyniaVulvodynia alsoalso knownknown asas:: • “essential” vulvodynia • generalized vulvar dysesthesia Dysesthesia Unpleasant,Unpleasant, abnormalabnormal sensationsensation examplesexamples include:include: -

Anterior Abdominal Wall
Abdominal wall Borders of the Abdomen • Abdomen is the region of the trunk that lies between the diaphragm above and the inlet of the pelvis below • Borders Superior: Costal cartilages 7-12. Xiphoid process: • Inferior: Pubic bone and iliac crest: Level of L4. • Umbilicus: Level of IV disc L3-L4 Abdominal Quadrants Formed by two intersecting lines: Vertical & Horizontal Intersect at umbilicus. Quadrants: Upper left. Upper right. Lower left. Lower right Abdominal Regions Divided into 9 regions by two pairs of planes: 1- Vertical Planes: -Left and right lateral planes - Midclavicular planes -passes through the midpoint between the ant.sup.iliac spine and symphysis pupis 2- Horizontal Planes: -Subcostal plane - at level of L3 vertebra -Joins the lower end of costal cartilage on each side -Intertubercular plane: -- At the level of L5 vertebra - Through tubercles of iliac crests. Abdominal wall divided into:- Anterior abdominal wall Posterior abdominal wall What are the Layers of Anterior Skin Abdominal Wall Superficial Fascia - Above the umbilicus one layer - Below the umbilicus two layers . Camper's fascia - fatty superficial layer. Scarp's fascia - deep membranous layer. Deep fascia : . Thin layer of C.T covering the muscle may absent Muscular layer . External oblique muscle . Internal oblique muscle . Transverse abdominal muscle . Rectus abdominis Transversalis fascia Extraperitoneal fascia Parietal Peritoneum Superficial Fascia . Camper's fascia - fatty layer= dartos muscle in male . Scarpa's fascia - membranous layer. Attachment of scarpa’s fascia= membranous fascia INF: Fascia lata Sides: Pubic arch Post: Perineal body - Membranous layer in scrotum referred to as colle’s fascia - Rupture of penile urethra lead to extravasations of urine into(scrotum, perineum, penis &abdomen) Muscles . -

OBGYN Outpatient Surgery Coding
OBGYN Outpatient Surgery Coding Anatomy Anatomy • Hyster/o – uterus, womb • Uter/o – uterus, womb • Metr/o – uterus, womb • Salping/o – tube, usually fallopian tube • Oophor/o – ovary • Ovari/o - ovary Terminology • Colpo – vagina • Cervic/o – cervix, lower part of the uterus, the “neck” • Episi/o – vulva • Vulv/o – vulva • Perine/o – the space between the anus and vulva Hysterectomy • A hysterectomy is an operation to remove a woman's uterus. • A woman may have a hysterectomy for different reasons, including: • Uterine fibroids that cause pain • bleeding, or other problems. • Uterine prolapse, which is a sliding of the uterus from its normal position into the vaginal canal. Hysterectomy • There are around 30 hysterectomy CPT codes. • To find the correct code you have to first check: • the surgical approach and • extent of the procedure. Surgical Approaches • Abdominal – the uterus is removed via an incision in the lower abdomen • Vaginal – the uterus is removed via an incision in the vagina • Laparoscopic – the procedure is performed using a laparoscope , inserted via several small incisions in the body. • Their are also CPT codes for laparoscopic-assisted vaginal approach. In this procedure ,the scope is inserted via a small incisions in the vagina. Extent of Procedure • Total hysterectomy: It includes laparoscopically detaching the entire uterine cervix and body from the surrounding supporting structures and suturing the vaginal cuff. It includes bivalving, coring, or morcellating the excised tissues, as required. The uterus is then removed through the vagina or abdomen. • Subtotal, partial or supracervical hysterectomy: It is the removal of the fundus or op portion of the uterus only, leaving the cervix in place. -

SJH Procedures
SJH Procedures - Gynecology and Gynecology Oncology Services New Name Old Name CPT Code Service ABLATION, LESION, CERVIX AND VULVA, USING CO2 LASER LASER VAPORIZATION CERVIX/VULVA W CO2 LASER 56501 Destruction of lesion(s), vulva; simple (eg, laser surgery, Gynecology electrosurgery, cryosurgery, chemosurgery) 56515 Destruction of lesion(s), vulva; extensive (eg, laser surgery, Gynecology electrosurgery, cryosurgery, chemosurgery) 57513 Cautery of cervix; laser ablation Gynecology BIOPSY OR EXCISION, LESION, FACE AND NECK EXCISION/BIOPSY (MASS/LESION/LIPOMA/CYST) FACE/NECK General, Gynecology, Plastics, ENT, Maxillofacial BIOPSY OR EXCISION, LESION, FACE AND NECK, 2 OR MORE EXCISE/BIOPSY (MASS/LESION/LIPOMA/CYST) MULTIPLE FACE/NECK 11102 Tangential biopsy of skin (eg, shave, scoop, saucerize, curette); General, Gynecology, single lesion Aesthetics, Urology, Maxillofacial, ENT, Thoracic, Vascular, Cardiovascular, Plastics, Orthopedics 11103 Tangential biopsy of skin (eg, shave, scoop, saucerize, curette); General, Gynecology, each separate/additional lesion (list separately in addition to Aesthetics, Urology, code for primary procedure) Maxillofacial, ENT, Thoracic, Vascular, Cardiovascular, Plastics, Orthopedics 11104 Punch biopsy of skin (including simple closure, when General, Gynecology, performed); single lesion Aesthetics, Urology, Maxillofacial, ENT, Thoracic, Vascular, Cardiovascular, Plastics, Orthopedics 11105 Punch biopsy of skin (including simple closure, when General, Gynecology, performed); each separate/additional lesion -

Outpatient Surgical Procedures – Site of Service: CPT/HCPCS Codes
UnitedHealthcare® Commercial Policy Appendix: Applicable Code List Outpatient Surgical Procedures – Site of Service: CPT/HCPCS Codes This list of codes applies to the Utilization Review Guideline titled Effective Date: August 1, 2021 Outpatient Surgical Procedures – Site of Service. Applicable Codes The following list(s) of procedure and/or diagnosis codes is provided for reference purposes only and may not be all inclusive. The listing of a code does not imply that the service described by the code is a covered or non-covered health service. Benefit coverage for health services is determined by the member specific benefit plan document and applicable laws that may require coverage for a specific service. The inclusion of a code does not imply any right to reimbursement or guarantee claim payment. Other Policies and Guidelines may apply. This list contains CPT/HCPCS codes for the following: • Auditory System • Female Genital System • Musculoskeletal System • Cardiovascular System • Hemic and Lymphatic Systems • Nervous System • Digestive System • Integumentary System • Respiratory System • Eye/Ocular Adnexa System • Male Genital System • Urinary System CPT Code Description Auditory System 69100 Biopsy external ear 69110 Excision external ear; partial, simple repair 69140 Excision exostosis(es), external auditory canal 69145 Excision soft tissue lesion, external auditory canal 69205 Removal foreign body from external auditory canal; with general anesthesia 69222 Debridement, mastoidectomy cavity, complex (e.g., with anesthesia or more -

An International Urogynecological Association (IUGA)
Received: 18 December 2017 | Accepted: 18 December 2017 DOI: 10.1002/nau.23508 TERMINOLOGY An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the assessment of sexual health of women with pelvic floor dysfunction Rebecca G. Rogers MD1 | Rachel N. Pauls MD2 | Ranee Thakar MD3 | Melanie Morin PhD4 | Annette Kuhn MD5 | Eckhard Petri Dd, PhD6 | Brigitte Fatton MD7 | Kristene Whitmore MD8 | Sheryl Kinsberg PhD9 | Joseph Lee MBChB, FRANZCOG10 1 Dell Medical School, University of Texas, Austin, Texas 2 TriHealth Good Samaritan Hospital, Cincinnati, Ohio 3 Croydon University Hospital Croydon, London, United Kingdom 4 Universite de Sherbrooke, Montreal, Quebec, Canada 5 University Teaching Hospital Berne (Inselspital), Bern, Switzerland 6 University of Greifswald, Schwerin, Germany 7 University Hospital Nîmes, Nimes, Languedoc-Roussillon, France 8 Drexel University College of Medicine, Philadelphia, Pennsylvania 9 Case Western Reserve University, Cleveland, Ohio 10 University of New South Wales, St Vincents Hospital, Sydney, New South Wales, Australia Correspondence Aims: The terminology in current use for sexual function and dysfunction in women Rebecca G. Rogers, Department of Women's Health, 1301 W 38th Street, with pelvic floor disorders lacks uniformity, which leads to uncertainty, confusion, Suit705, Dell Medical School, University and unintended ambiguity. The terminology for the sexual health of women with of Texas, Austin, TX 78705. pelvic floor dysfunction needs to be collated in a clinically-based consensus report. Email: [email protected] Methods: This report combines the input of members of the Standardization and Terminology Committees of two International Organizations, the International Urogynecological Association (IUGA), and the International Continence Society (ICS), assisted at intervals by many external referees.