Hypohydration During Prolonged Exercise in the Heat
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

TERMS and CONDITIONS Standard Chartered Kuala Lumpur Marathon 2019 (“SCKLM 2019”) Priority Sign up Campaign
TERMS AND CONDITIONS Standard Chartered Kuala Lumpur Marathon 2019 (“SCKLM 2019”) Priority Sign Up Campaign Campaign 1.0 The Standard Chartered Bank Malaysia Berhad and Standard Chartered Saadiq Berhad (“the Bank”) Standard Chartered KL Marathon 2019 Priority Sign-Up Campaign (“Campaign”) will run from 15 January 2019 to 15 February 2019, inclusive of both dates (“Campaign Period”). 2.0 This campaign includes accounts under Standard Chartered Bank Malaysia Berhad (“SCBMB”) and also Standard Chartered Saadiq Berhad (“SCSB”). 3.0 By participating in this Campaign, participants agree to be bound by all the terms and conditions below. This Campaign terms must be read together with the product terms and the relevant banking agreements. If there are any inconsistencies between these terms and the relevant product terms and the banking agreement, these terms shall prevail limited only to the inconsistencies. Eligibility 4.0 This campaign is open to New-to-Bank (“NTB”) clients of the Bank who fulfil the following conditions (“Successful Sign-Up”): 4.1 Successfully establish a new Priority relationship where the NTB needs to maintain a minimum of MYR250,000 in deposits and/or investments as a Primary account holder during the Campaign Period. The Priority banking terms and conditions are available at https://www.sc.com/my/priority/tnc/; 4.2 Individuals over the age of 18 years. A NTB client is defined as an individual who starts a relationship with the Bank during the Campaign Period and does not include an individual who maintains or had maintained a banking relationship with the Bank within the past twelve (12) months from the date of commencement of the Campaign Period. -

The Bull Runner Magazine When She Bagged 2Nd Place for Women in Standard Chartered Do Not Necessarily Represent You Won One Pair of You Featured Inspired Me a Lot
Runner Bull The Runner Bull The [RACES] House, Glorietta 4. www. Call 491-5105 / 413-8083. avonkgbcphilippines.com www.thepinkrun.com SEPTEMBER 3: TAKBO! BREAST 17: THE BLUEWATER Hill, 3/5/10k. Call Nikki FRIENDS, Ultra, 3/5/10k. DAY SPA FUN RUN, SM 421-1213/ 0922-8151942 11: THE FS RUN (The Call 502-4260/ 0917- MOA. Call 703-1736 27: 1ST CEBU ULTRA- Fort Striders Club), 8138149 Bonifacio Global City, 24: ADIDAS KING OF MARATHON, Cebu City. 5/10/16k. Call 896-1954 3: TRAIL CALL RUN, THE Road, Bonifacio [email protected] Timberland, San Mateo, Global City, 5/10/21k. ST 28: MILO MARATHON - 12: 1 FINEX FUN RUN, 3/5/10k. Call 0927- Register at adidas Stores, CDO.Call Rudy Biscocho SM MOA, 3/5/10k. Call 3409023 RUNNR Store, BHS. 811-4052. finex.org.ph 0922-8367992 9: SOUTHRIDGE RUN, 24: MAYON TRAIL RUN, 28: KNOWLEDGE 12: THRILL OF THE West Gate Alabang, Legazpi City, 21k. www. CHANNEL TRAIL, Bohol, 11/22k. 1/5/10k. mayontrailrun.com www.thrillofthetrail.ph KARUNUNGAN 9: 1ST PAU MT. PINATUBO 31: MILO MARATHON 2010, Meralco, Ortigas, 12: MAKILING 50K TRAIL CHALLENGE, - Puerto Princesa. Call 3/5/10/15k. Call 983-5204/ CHALLENGE 9, U.P. Los Sta. Juliana, Capas, Tarlac Rudy Biscocho 0922- 983-2903, 0917-6256517 Baños, 5/10/16k. Call to Mt. Pinatubo Crater. 8367992 0906-4251387 Call 497-6442 [CLINICS] 12: MILO MARATHON 10: RUN FOR PASIG NOVEMBER MON/WED/FRI, 6:00 pm: - Tagbilaran. Call Rudy RIVER, 3/5/10/21k. 3k- Biscocho 0922-8367992 7: ATENEO BLUE RACE, RUNWALKING CLINIC, SM MOA, 5k- Luneta, ADMU, 3/5/7.5k, Call Philsports Complex 19: MILO MARATHON 10k- Bonifacio Global 0922-8330928, (ULTRA). -

Abstract Introduction
Global Media Journal – Malaysian Edition Volume 6, Issue 2, 2016 ISSN : 2231-9948 THE NUPTIALS OF SOCIAL AND TRADITIONAL MEDIA – WHY THIS SOPHISTICATED MIXED MARRIAGE IS WORTH MONITORING IN THE LONG-TERM Elliza Abdul Rahim Essence Communications Pusat Dagangan, Phileo Damansara 1, Selangor, Malaysia. [email protected] ABSTRACT For companies in Malaysia who want to stay ahead, monitoring their traditional media is no longer an only option. It is imperative that attention is paid to the implications of the heavy activity on the Internet via portals and social networking sites. Neglect would lead to a handicap of a swift response in real time which is a necessity in this age of nanotechnology. Integrating traditional and social media monitoring elevates management of information to a level that allows organizations to document, analyze and place a numeric value to their public relation efforts. Therefore, the objective of this paper is to highlight the activities reflecting the media monitoring based on a few samples of case studies and at the end emphasizes on the worth of mixed marriage of traditional and social media. Keywords: Mixed marriage; traditional; social media; monitoring and social sites. INTRODUCTION This country has been advocating mixed marriages since Adam. We marry the Malay and English language to make Manglish – much to the dismay of the English Language purist. We marry a plethora of food, customs and traditions to create the unique Malaysians we are today. It should not come as a surprise that there is an important 1 Global Media Journal – Malaysian Edition Volume 6, Issue 2, 2016 ISSN : 2231-9948 public relations marriage quietly being negotiated in the media arena that we need to be aware of. -

Cover Nov 11-01
Volume: 06 | Issue: 04 | April 2015 MTBiz 1 ARTICLE OF THE MONTH An Account of Internaonal Trade of Bangladesh Introducon Industrial raw materials leads the total import volume of the Bangladesh started its internaonal trade since its independence country. From 2007-08 to 2013-14, import of industrial raw and it has been showing improvement in its trade balance materials has been following a posive trend and volume has since then. Though growth was bit flat in its early decades, the been geng bigger and bigger, which is a posive sign in favor improvement excelled since 1990s with the incepon of trade of development of the country. Petrolium and capital machinary liberalizaon. Bangladesh did very well in export, especially in also has been increasing over the years. These three secotrs of RMG, Shrimp & Fish, Leather and Jute & Jute goods sectors. Rise in import are very crucial for economic development and boosng imports of capital machinery and industrial raw materials, instead the export in the long run. of food and consumable items, is a visible trend in Bangladesh now. This arcle is an aempt to sketch the trajectory of Bangladesh in internaonal trade. It depicts the trend of growth in major sectors of export and import. It progresses with a comparave view of foreign trade and elucidates the trade balance of the country. This arcle also depicts the globally leading countries of internaonal trade and their trends. It ends up with a comparave analysis of Bangladesh in global and South Asian contexts. Import data of Bangladesh has been obtained from weekly publicaon of the country’s Central Bank. -

35153 Stanchart R&A 2004 0 Copy
35153 StanChart R&A 2004 C copy 8/3/05 10:01 AM Page 1 Annual Report and Accounts 2004 Standard Chartered Chartered Standard Annual Report and Accounts 2004 ds Leading the way ing ugh in Asia, Africa and – the andard of eye the Middle East rence. ct: ered.com 35153 StanChart R&A 2004 C copy 8/3/05 9:59 AM Page 2 Front Cover: Standard Chartered employs 33,000 people in over Thomas Migwi Kamau breaks the tape 550 locations serving 56 countries and territories to win the 2004 Standard Chartered Hong Kong Marathon across the Asia Pacific region, South Asia, the Middle Mr Kamau, from Kenya, set a personal best time in the 8th annual Standard Chartered Hong Kong East, Africa, the United Kingdom and the Americas. Marathon. The event, which hosted a record 24,000 runners in 2004, is a signature international sporting It is one of the world’s most international banks with event, attracting world-class runners from all corners employees representing 80 nationalities. of the globe. Standard Chartered serves both Consumer and Wholesale Banking customers, is well established in growth markets and aims to be The Right Partner for its customers. The Bank is trusted across its network for its standards of governance and its commitment to making a difference in the communities in which it operates. Leading the Way Leading the Way represents our strategic intent. It is the framework within which we make our strategic and operational decisions. In the context of this framework, each country and business decides its role and determines what actions are required to achieve the Strategic Intent. -

AIMS CALENDAR January 2010 - March 2011
AIMS CALENDAR January 2010 - March 2011 2010 16 Jan 2010 K inmen Marathon M/H TPE Taroko Gorge Marathon November Woon Kong Lee Post Office Box 47-110 Taipei, Taiwan, R.O.C Tel: 886 2 2585 5659 Fax: 886 2 2599 6716 Email: [email protected] Web: www.sportsnet.org.tw 17 Jan 2010 Standard Chartered MumBai Marathon IND M Hugh Jones Procam International, 14, St. James Court, Marine Drive, Mumbai - 20 Tel: 91 22 2202 02 84 Fax: 91 22 2202 5112 Email: [email protected] Web: www.procamintl.com Part of The Greatest Race on Earth 17 Jan 2010 China Coast Marathon and Half M/H H K G Catherine Leonard AVOHK, P.O. Box 28893, Gloucester Road Post Office, Wanchai Tel: 852 9038 5460 Email: [email protected] Web: www.avohk.org 17 Jan 2010 Maraton Internacional de Culiacan M E X M Juan Antonio Lopez Garibaldi Constitución 175 pte., Colonia Almada C.P. 80200, Culiacán, Sinaloa Tel: 667 712 4710 Fax: 667 713 9116 Email: [email protected] Web: www.maratonculiacan.org.mx 20 Jan 2010 Corrida de Sao Sebastiao 10km BR A R Joao Traven Rua Felix Pacheco 150 BL C-Apt 102 Leblon, Rio - RJ Tel: 55 21 2223 2773 Fax: 55 21 2223 3073 Email: [email protected] Web: www.corridadesaosebastiao.com.br 22 Jan 2010 Standard Chartered Dubai Marathon UAE M Peter Connerton P.O. Box 57176, Dubai Tel: 97 14 367 1062 Fax: 97 14 367 2524 Email: [email protected] Web: www.dubaimarathon.org 24 Jan 2010 K hon K aen Marathon THA M Apsit Rujikeatkamjorm Khon Kaen University Alumni AssociatIon, Khon Kaen University, 123/101 Moo 16, Tumbol Nai Muang, Amphur Muang, Khon Kaen 40002 Tel: 66 43 202 750 / 66 43 202 388 Fax: 66 43 202 750 / 66 43 202 388 Email: [email protected] Web: www.khonkaenmarathon.com 24 Jan 2010 Maui Ocean F ront Marathon & Half USA M Les Wright 2480 S. -

Qualifying Events WCH 2011 in 2010
13th IAAF World Championships in Athletics Daegu, KOR List of Qualifying Marathon Races in 2011 As at 1 April 2011 Country City Name of Race ARG Buenos Aires Buenos Aires Marathon AUS Blacktown Cities M7 Marathon AUS Bunbury Bunbury Marathon AUS Canberra Canberra Marathon AUS Melbourne Samsung Melbourne Marathon AUS Perth Perth Marathon AUS Perth Perth City to Surf Marathon AUS Rottnest/Perth Rottnest Marathon AUS Southport Gold Coast Airport Marathon AUS Sydney Sydney Marathon AUT Linz Linz Int'l Marathon AUT Salzburg AMREF Salzburg Marathon AUT Vienna Vienna City Marathon BAH Nassau Marathon Bahamas BAR Bridgetown Run Barbados Marathon BEL Antwerp Antwerp Marathon BER Hamilton Bermuda Marathon BOT Gaborone Gaborone City Marathon BOT Phikwe Botswana Phikwe Natl. Marathon Championships BRA Florianopolis Santa Catalina Marathon BRA Porto Alegre Porto Alegre Marathon BRA Rio de Janeiro Rio City Marathon CAN Calgary, AB Calgary Marathon CAN Edmonton, MB Edmonton Marathon CAN Kelowna Okanagan Marathon CAN Montreal Marathon International de Montreal CAN Ottawa ING Ottawa Marathon CAN Toronto Toronto Waterfront CAN Vancouver BMO Vancouver Marathon CAN Victoria Royal Victoria Marathon CAN Niagara Falls, ON Niagara Falls International Marathon CAY Georgetown Cayman Islands Marathon CHI Santiago Maratón De Santiago CHN Beijing Beijing International Marathon CHN Dalian Dalian Marathon CHN Hangzhou Hangzhou International Marathon CHN Shanghai Toray Cup Shanghai Intl Mthn CHN Xiamen Xiamen Int'l Marathon CRC San José Maraton Intl de Costa Rica CRC -
2003 Annual Review
30102 StanChart AnRvw Covers 7/5/2004 3:51 pm Page 1 Annual Review 2003 seeing is believing Standard Chartered Chartered Standard Annual Review 2003 Standard Chartered aims to raise funds to restore sight to one million people. © Standard Chartered PLC Our Seeing is Believing global fundraising campaign has already raised March 2004 US$1,400,000, enough to restore the sight of 56,000 people, twice our Registered Office: original target. 1 Aldermanbury Square The money raised will be administered by Sight Savers International and London EC2V 7SB other members of Vision 2020 ‘The Right to Sight’. Telephone +44 (0)20 7280 7500 Leading the way We would like to thank all our employees, customers, suppliers and www.standardchartered.com shareholders for their generous support. Principal Place of Business in Asia, Africa and Now we want to raise enough to restore a million eyesights. in Hong Kong: 32nd Floor, 4-4A Des Voeux Road, If you would like to support Seeing is Believing please contact: Genevieve Kotta, Community Relations Manager the Middle East Central, Hong Kong [email protected] Registered in England Telephone: +44 (0) 20 7280 7657 number: 966425 www.standardchartered.com 30102 StanChart AnRvw Covers 7/5/2004 3:51 pm Page 2 Front Cover: Standard Chartered employs 30,000 people in over The 2004 Standard Chartered Bank 500 locations serving 56 countries and territories Hong Kong Marathon. across the Asia Pacific Region, South Asia, the Standard Chartered has sponsored the Hong Kong Middle East, Africa, the United Kingdom and the Marathon since 1997. This year 24,000 runners took Americas. -

View Annual Report
Annual Report and Accounts 2005 Leading the way in Asia, Africa and the Middle East Contents Leading the way Business highlights 1 Group at a Glance 2 in Asia, Africa and Chairman’s Statement 4 Chief Executive’s Review 6 Business Review 12 Financial Review 22 the Middle East Board of Directors 38 Senior Management 40 Report of the Directors 41 Drawing on over 150 years of international Corporate Governance 44 Directors’ Remuneration Report 49 banking experience, Standard Chartered is Statement of Directors’ actively driving value creation in its markets Responsibility 62 with the right strategy for growth. Auditor’s Report 63 Income Statement 64 Combining deep local knowledge with Balance Sheet 65 global capability, Standard Chartered offers Notes to the Accounts 69 Supplementary Financial innovative, award-winning financial products Information 136 and services, in many of the world’s fastest Principal Group Addresses 140 growing markets. Shareholder Information 142 Index 144 Awards 145 Employing almost 44,000 people, representing 89 nationalities in over 1,200 locations and engaged in a broad range of Comsumer and Wholesale Banking activities, Standard Chartered’s strength lies in breadth, diversity and balance. Standard Chartered is committed to being The Right Partner to all its stakeholders. Across its network the Bank is trusted for its outstanding standards of governance and its commitment to making a difference to the communities in which it operates. The Business Review section of this Report combines information on corporate responsibility and people strategies, along with operating highlights, into a balanced account of the year at Standard Chartered. From our earliest branches in Africa, to our state-of-the-art priority banking centre in Taipei, our commitment to the customers, employees and communities in the markets in which we operate makes us one of the world’s best international banks. -

LIST of WORLD ATHLETICS CERTIFIED MARATHON and HALF MARATHON COURSES Updated 25 June 2021 (Includes 25Km and 30Km Courses Valid for "Marathon" World Rankings)
LIST OF WORLD ATHLETICS CERTIFIED MARATHON AND HALF MARATHON COURSES Updated 25 June 2021 (includes 25km and 30km courses valid for "Marathon" World Rankings) Country City Name of Race Event Drop and Separation ALB Tirana Tirana Half Marathon Half Marathon Comp. Rule 31.21 compliant ALG Alger Marathon international d'Alger Marathon Comp. Rule 31.21 compliant ALG Bejaia Semi marathon international de la ville de Bejaia Half Marathon Comp. Rule 31.21 compliant ARG Buenos Aires 21K Buenos Aires Half Marathon Comp. Rule 31.21 compliant ARG Buenos Aires Maratón Int’l de la Ciudad de Buenos Aires Marathon Comp. Rule 31.21 compliant ARG Mendoza Maratón Int’l de Mendoza Marathon Downhill (-10.7 m/km), Separation >50% ARG Santa Rosa Maratón Int’l “A Pampa Taviesa” Marathon Comp. Rule 31.21 compliant ARM Yerevan Yerevan Marathon Marathon Comp. Rule 31.21 compliant ARU Aruba (Eagle Beach) KLM Aruba Marathon Marathon Comp. Rule 31.21 compliant AUS Adelaide Adelaide Westpac City-Bay Half Marathon Half Marathon Comp. Rule 31.21 compliant AUS Adelaide Adelaide Marathon Festival - Half Marathon Half Marathon Comp. Rule 31.21 compliant AUS Adelaide Adelaide Marathon Festival - Marathon Marathon Comp. Rule 31.21 compliant AUS Airlie Beach, Queensland Airlie Beach Marathon Festival - Half Marathon Half Marathon Comp. Rule 31.21 compliant AUS Airlie Beach, Queensland Airlie Beach Marathon Festival - Marathon Marathon Comp. Rule 31.21 compliant AUS Albury Murray River Half Marathon Half Marathon Comp. Rule 31.21 compliant AUS Albury Murray River Marathon Marathon Comp. Rule 31.21 compliant AUS Alice Springs Alice Springs Half Marathon Half Marathon Comp. -
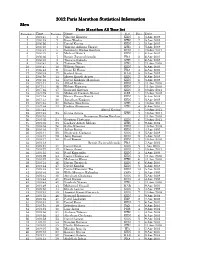
2012 Paris Marathon Marathon Marathon Statistical Statistical Statistical Information Information Information
2020201220 121212 Paris Marathon Statistical Information Men Paris Marathon All Time list Performance Time Performer Name Nat Place Date 1 2:05:47 1 Vincent Kipruto KEN 1 5 Apr 2009 2 2:06:15 2 Bazu Worku ETH 2 5 Apr 2009 3 2:06:26 3 David Kiyeng KEN 3 5 Apr 2009 4 2:06:30 4 Yemane Adhane Tsegay ETH 4 5 Apr 2009 5 2:06:31 5 Benjamin Kiptoo Koulum KEN 1 10 Apr 2011 6 2:06:33 6 Michael Rotich KEN 1 6 Apr 2003 7 2:06:36 7 Benoit Zwierzchiewski FRA 2 6 Apr 2003 8 2:06:40 8 Tsegaye Kebede ETH 1 6 Apr 2008 9 2:06:41 9 Tadesse Tola ETH 1 11 Apr 2010 10 2:06:47 10 Wilson Onsare KEN 3 6 Apr 2003 11 2:06:48 11 Driss El Himer FRA 4 6 Apr 2003 12 2:06:48 12 Rachid Kisri MAR 5 5 Apr 2009 13 2:06:50 13 Moses Kimeli Arusei KEN 2 6 Apr 2008 14 2:06:53 14 David Kipkorir Ma ndago KEN 6 5 Apr 2009 15 2:07:11 15 Alfred Kering KEN 2 11 Apr 2010 16 2:07:13 16 Wilson Kipsang KEN 3 11 Apr 2010 17 2:07:16 17 Bernard Kipyego KEN 2 10 Apr 2011 18 2:07:19 18 Mubarak Hassan Shami QAT 1 15 Apr 2007 19 2:07:24 19 Hosea Kiprop Rotich KEN 3 6 Apr 2008 20 2:07:31 20 Jonathan Kipkorir KEN 7 5 Apr 2009 21 2:07:33 21 Eshetu Wendimu ETH 3 10 Apr 2011 22 2:07:34 22 Gudisa Shentema ETH 4 6 Apr 2008 23 2:07:41 Alfred Kering 4 10 Apr 2011 24 2:07:43 23 Girma Assefa ETH 5 10 Apr 2011 25 2:08:01 Benjamin Kiptoo Koulum 4 11 Apr 2010 26 2:08:02 24 Stephen Chebogut KEN 6 10 Apr 2011 27 2:08:03 25 Gashaw Asfaw Melese ETH 1 9 Apr 2006 28 2:08:04 26 Salim Kipsang KEN 1 10 Apr 2005 29 2:08:10 27 Julius Rutto KEN 1 4 Apr 1999 30 2:08: 11 28 Shadrack Kiplagat KEN 8 5 Apr 2009 31 2:08:12 -

LIST of IAAF CERTIFIED MARATHON and HALF MARATHON COURSES Updated 5 October 2018 (Includes 25Km and 30Km Courses Valid for Marathon World Rankings)
LIST OF IAAF CERTIFIED MARATHON AND HALF MARATHON COURSES Updated 5 October 2018 (includes 25km and 30km courses valid for Marathon World Rankings) Country City Name of Race Event Drop and Separation ALB Tirana Tirana Half Marathon Half Marathon IAAF rule compliant ALG Alger Marathon international d'Alger Marathon IAAF rule compliant ALG Bejaia Semi marathon international de la ville de Bejaia Half Marathon IAAF rule compliant ARG Buenos Aires 21K Buenos Aires Half Marathon IAAF rule compliant ARG Buenos Aires Maratón Int’l de la Ciudad de Buenos Aires Marathon IAAF rule compliant ARG Mendoza Maratón Int’l de Mendoza Marathon Downhill, Separation > 50% ARG Santa Rosa Maratón Int’l “A Pampa Taviesa” Marathon IAAF rule compliant ARG Villa Carlos Paz 21K Media Maratón Carlos Paz Half Marathon IAAF rule compliant ARM Yerevan Yerevan Half Marathon Half Marathon IAAF rule compliant ARU Aruba (Eagle Beach) KLM Aruba Marathon Marathon IAAF rule compliant AUS Adelaide Adelaide Half Marathon Half Marathon IAAF rule compliant AUS Adelaide Adelaide Marathon Marathon IAAF rule compliant AUS Albury Murray River Half Marathon Half Marathon IAAF rule compliant AUS Albury Murray River Marathon Marathon IAAF rule compliant AUS Alice Springs Alice Springs Half Marathon Half Marathon IAAF rule compliant AUS Alice Springs Alice Springs Marathon Marathon IAAF rule compliant AUS Bendigo Bendigo Bank Half Marathon Half Marathon IAAF rule compliant AUS Blacktown Westlink M7 Blacktown Half Marathon Half Marathon IAAF rule compliant AUS Blacktown Westlink M7 Cities