Overbars Structural Reinvestigation of Getchellite As0.98Sb1.02S3.00
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stibnite Gold Project
STIBNITE GOLD PROJECT PAYETTE & BOISE NATIONAL FORESTS INTERMOUNTAIN REGION August 22, 2018 STIBNITE MINING DISTRICT, PAYETTE NATIONAL FOREST, KRASSEL RANGER DISTRICT Regarding Locatable Minerals , 36 CFR 228, Subpart A: requires the Forest Service to: "Where conflicting interests Respond to a mining plan, evaluate that plan must be reconciled, the Consider requirements to minimize adverse effects to the extent feasible question shall always be Comply with applicable laws, regulations and answered from the stand- standards for environmental protection point of the greatest good of Assure appropriate reclamation Further respond by following National Environmental the greatest number in the Policy Act (NEPA) processes. long run." - James Wilson, Secretary Per 36 CFR 228.4, the Forest Service is required to take of Agriculture, 1905 in a letter to Gifford action to ensure that “operations are conducted so as, Pinchot, 1st Chief of the Forest Service. where feasible, to minimize adverse environmental impacts on National Forest surface resources. 1 Table of Contents Project Context STIBNITE GOLD Project Location 4 Mining History at Stibnite 5 PROJECT Who is Midas Gold 6 Stibnite Plan of Operations PAYETTE & BOISE NATIONAL Proposed Mining Plan of FOREST - INTERMOUNTAIN REGION Operations 7 Open Pit Mining 8 Anadromous Fish - Tunnel 9 Inventoried Roadless Areas - Why is this project of interest to many Access and Powerline 10 people and organizations? Facilities During Mining Operations 10 5 million recoverable ounces of gold x -

Theoretical Studies on As and Sb Sulfide Molecules
Mineral Spectroscopy: A Tribute to Roger G. Bums © The Geochemical Society, Special Publication No.5, 1996 Editors: M. D. Dyar, C. McCammon and M. W. Schaefer Theoretical studies on As and Sb sulfide molecules J. A. TOSSELL Department of Chemistry and Biochemistry University of Maryland, College Park, MD 20742, U.S.A. Abstract-Dimorphite (As4S3) and realgar and pararealgar (As4S4) occur as crystalline solids con- taining As4S3 and As4S4 molecules, respectively. Crystalline As2S3 (orpiment) has a layered structure composed of rings of AsS3 triangles, rather than one composed of discrete As4S6 molecules. When orpiment dissolves in concentrated sulfidic solutions the species produced, as characterized by IR and EXAFS, are mononuclear, e.g. ASS3H21, but solubility studies suggest trimeric species in some concentration regimes. Of the antimony sulfides only Sb2S3 (stibnite) has been characterized and its crystal structure does not contain Sb4S6 molecular units. We have used molecular quantum mechanical techniques to calculate the structures, stabilities, vibrational spectra and other properties of As S , 4 3 As4S4, As4S6, As4SIO, Sb4S3, Sb4S4, Sb4S6 and Sb4SlO (as well as S8 and P4S3, for comparison with previous calculations). The calculated structures and vibrational spectra are in good agreement with experiment (after scaling the vibrational frequencies by the standard correction factor of 0.893 for polarized split valence Hartree-Fock self-consistent-field calculations). The calculated geometry of the As4S. isomer recently characterized in pararealgar crystals also agrees well with experiment and is calculated to be about 2.9 kcal/mole less stable than the As4S4 isomer found in realgar. The calculated heats of formation of the arsenic sulfide gas-phase molecules, compared to the elemental cluster molecules As., Sb, and S8, are smaller than the experimental heats of formation for the solid arsenic sulfides, but shown the same trend with oxidation state. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

40 Common Minerals and Their Uses
40 Common Minerals and Their Uses Aluminum Beryllium The most abundant metal element in Earth’s Used in the nuclear industry and to crust. Aluminum originates as an oxide called make light, very strong alloys used in the alumina. Bauxite ore is the main source aircraft industry. Beryllium salts are used of aluminum and must be imported from in fluorescent lamps, in X-ray tubes and as Jamaica, Guinea, Brazil, Guyana, etc. Used a deoxidizer in bronze metallurgy. Beryl is in transportation (automobiles), packaging, the gem stones emerald and aquamarine. It building/construction, electrical, machinery is used in computers, telecommunication and other uses. The U.S. was 100 percent products, aerospace and defense import reliant for its aluminum in 2012. applications, appliances and automotive and consumer electronics. Also used in medical Antimony equipment. The U.S. was 10 percent import A native element; antimony metal is reliant in 2012. extracted from stibnite ore and other minerals. Used as a hardening alloy for Chromite lead, especially storage batteries and cable The U.S. consumes about 6 percent of world sheaths; also used in bearing metal, type chromite ore production in various forms metal, solder, collapsible tubes and foil, sheet of imported materials, such as chromite ore, and pipes and semiconductor technology. chromite chemicals, chromium ferroalloys, Antimony is used as a flame retardant, in chromium metal and stainless steel. Used fireworks, and in antimony salts are used in as an alloy and in stainless and heat resisting the rubber, chemical and textile industries, steel products. Used in chemical and as well as medicine and glassmaking. -

Using 2D Gis to Assist 3D Modelling of the Zarshuran Gold Deposit, Iran
Asadi, Hooshang USING 2D GIS TO ASSIST 3D MODELLING OF THE ZARSHURAN GOLD DEPOSIT, IRAN * Hooshang ASADI HARONI, Edmund SIDES, Kiiza NGONZI International Institute for Aerospace Survey and Earth Sciences (ITC), The Netherlands * Ministry of Higher Education, Tehran, Iran harouni @itc.nl [email protected] Technical Commission Session Themes TC VII-8 KEY WORDS: Spatial data, Integration, Geology, Geophysics, GIS, Data mining, Information extraction ABSTRACT The Zarshuran gold deposit in NW Iran is an area of historic mining for gold and arsenic with considerable potential for discovery of economic gold mineralisation. Geological, geochemical and geophysical data, collected by the Ministry of Mines and Metals were compiled and analysed in a 2-dimensional (2D) GIS. This resulted in the definition of major structural features, and lithological units, that control the gold mineralisation. Spatial modelling and interpretation of the geochemical and geophysical data showed that the mineralization is mainly controlled by chemically-reactive Precambrian carbonates and black shales, extending in a NW-SE direction, and also by NE-SW high angle faults and their intersections with NW-SE structures. The results obtained, from the 2D GIS analysis, were used in the initial phase of the construction and validation of the 3-dimensional (3D) models used for resource estimation. Comparison of the statistical analyses of geochemical data in soils and in drillcore indicated enhanced concentrations of gold in soils at surface, due to residual enrichment. An enrichment relationship was established based on interpretation of the cumulative frequency plots for gold in soil and drillcore samples. Based on this relationship the gold anomalies interpreted from the soil geochemical data were used to infer a resource potential, to a depth of 200m below surface, of 10Mt at an average grade of 0.2 g/t gold. -

REVISION 1 an Insight at the Inverse Transformation of Realgar Altered By
REVISION 1 An insight at the inverse transformation of realgar altered by light Giovanni Pratesi1,2 and Matteo Zoppi1* 1Museo di Storia Naturale - Sezione Mineralogia e Litologia, Università di Firenze, via La Pira 4, I- 50121 Firenze, Italy. 2Dipartimento di Scienze della Terra, Università di Firenze, via La Pira 4, I-50121 Firenze, Italy. * E-mail: [email protected] ABSTRACT The light-induced alteration of realgar and β-As4S4 is a well known phenomenon but still displays interesting aspects not completely explained. In the transformation from realgar to pararealgar the molecule As4S4 undergoes a structural modification, and ever since the initial studies two important issues have been highlighted: the influence of the oxygen and the reversibility of the process. The previous study on the reversibility of the altered β-As4S4 points out that this polymorph exhibits a dual behaviour. When the light-induced alteration occurs with the presence of the air, pararealgar and arsenolite, along with amorphous material, are the products, while if the air is not present β-As4S4 turns completely into pararealgar. Moreover, when annealing the altered material in the realgar stability fields (220 °C), in the first case pararealgar and amorphous material turn into stoichiometric alacranite, while in the second case the alteration is completely reversible. Similarly, the present study focuses the attention on the question if realgar, when altered by means of the light and when annealed, might behave as β-As4S4 does. These results display that the phenomenon is more complex. The alteration of realgar with the presence of the air yields pararealgar along with arsenolite, a small quantity of uzonite and amorphous material, and when the air is not present pararealgar is the only product. -

The Importance of Minerals in Coal As the Hosts of Chemical Elements: a Review
The importance of minerals in coal as the hosts of chemical elements: A review Robert B. Finkelmana,b, Shifeng Daia,c,*, David Frenchd a State Key Laboratory of Coal Resources and Safe Mining, China University of Mining and Technology, China b University of Texas at Dallas, Richardson, TX 75080, USA c College of Geoscience and Survey Engineering, China University of Mining and Technology (Beijing), Beijing 100083, China d PANGEA Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia *, Corresponding author: [email protected]; [email protected] Abstract Coal is a complex geologic material composed mainly of organic matter and mineral matter, the latter including minerals, poorly crystalline mineraloids, and elements associated with non- mineral inorganics. Among mineral matter, minerals play the most significant role in affecting the utilization of coal, although, in low rank coals, the non-mineral elements may also be significant. Minerals in coal are often regarded as a nuisance being responsible for most of the problems arising during coal utilization, but the minerals are also seen as a potentially valuable source of critical metals and may also, in some cases, have a beneficial effect in coal gasification and liquefaction. With a few exceptions, minerals are the major hosts of the vast majority of elements present in coal. In this review paper, we list more than 200 minerals that have been identified in coal and its low temperature ash, although the validity of some of these minerals has not been confirmed. Base on chemical compositions, minerals found in coal can be classified into silicate, sulfide and selenide, phosphate, carbonate, sulfate, oxide and hydroxide, and others. -

Orpiment As2s3 C 2001-2005 Mineral Data Publishing, Version 1
Orpiment As2S3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Monoclinic. Point Group: 2/m. Commonly in foliated columnar or fibrous aggregates, with cleavages as much as 60 cm across; may be reniform or botryoidal; also granular or powdery; rarely as prismatic crystals, to 10 cm. Twinning: On {100}. Physical Properties: Cleavage: Perfect on {010}, imperfect on {100}; cleavage lamellae are flexible. Tenacity: Sectile. Hardness = 1.5–2 VHN = n.d. D(meas.) = 3.49 D(calc.) = 3.48 Optical Properties: Transparent. Color: Lemon-yellow to golden or brownish yellow. Streak: Pale lemon-yellow. Luster: Resinous, pearly on cleavage surface. Optical Class: Biaxial (–). Pleochroism: In reflected light, strong, white to pale gray with reddish tint; in transmitted light, Y = yellow, Z = greenish yellow. Orientation: X = b; Z ∧ c = 2◦. Dispersion: r> v,strong. α = 2.4 (Li). β = 2.81 (Li). γ = 3.02 (Li). 2V(meas.) = 76◦ Anisotropism: Barely observable because of strong internal reflections. R1–R2: (400) 33.0–36.5, (420) 31.0–35.2, (440) 28.9–33.9, (460) 27.4–31.5, (480) 26.0–30.3, (500) 24.9–29.3, (520) 24.0–28.4, (540) 23.3–27.8, (560) 22.8–27.3, (580) 22.3–26.9, (600) 22.0–26.5, (620) 21.7–26.3, (640) 21.5–26.0, (660) 21.2–25.7, (680) 21.0–25.5, (700) 20.8–25.3 Cell Data: Space Group: P 21/n. a = 11.475(5) b = 9.577(4) c = 4.256(2) β =90◦41(5)0 Z=4 X-ray Powder Pattern: Baia Sprie (Fels˝ob´anya), Romania. -

10700 Orpiment, King's Yellow PY 39
10700 Orpiment, King's Yellow PY 39 Chemical composition: yellow sulphide of arsenic As2S3 The origin of the modern name is derived from the Latin term auripigmentum or auripigmento, literally meaning gold paint. Orpiment was once widely used, particularly in the East, but has now fallen into disuse because of its limited supply and because of its poisonous character. The principal sources in ancient times appear to have been in Hungary, Macedonia, Asia Minor and perhaps in various parts of Central Asia. There was a large deposit near Julamerk in Kurdistan. Current deposits of orpiment are in Romania, Hungary, Germany, Greece, France, Italy, Iran, Peru, China, Japan and the western United States. Orpiment occurs as a low temperature product in hydrothermal veins, as a volcanic sublimation product, as a hot spring deposit and in fire mines. It is often associated with stibnite, pyrite, realgar, calcite and gypsum. Orpiment occurs in many places but not in large quantities. Orpiment is usually described as a lemon or canary yellow or sometimes as a golden or brownish yellow with a fair covering power. Microscopically, orpiment is crystalline and may contain orange-red particles of realgar, to which it is closely related. The larger particles glisten by reflected light and have a waxy-looking surface. The toxicity of the arsenic sulfide pigments has been known since early times. The toxic properties of orpiment have been used to advantage to repel insects. Orpiment is said to be incompatible with lead- or copper-containing pigments. Orpiment is not stable in lime and therefore can not be used for fresco, a fact noted by Cennino Cennini inn the fifteenth century. -
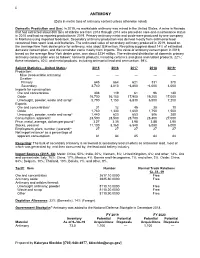
Antimony Data Sheet
22 ANTIMONY (Data in metric tons of antimony content unless otherwise noted) Domestic Production and Use: In 2019, no marketable antimony was mined in the United States. A mine in Nevada that had extracted about 800 tons of stibnite ore from 2013 through 2014 was placed on care-and-maintenance status in 2015 and had no reported production in 2019. Primary antimony metal and oxide were produced by one company in Montana using imported feedstock. Secondary antimony production was derived mostly from antimonial lead recovered from spent lead-acid batteries. The estimated value of secondary antimony produced in 2019, based on the average New York dealer price for antimony, was about $34 million. Recycling supplied about 14% of estimated domestic consumption, and the remainder came mostly from imports. The value of antimony consumption in 2019, based on the average New York dealer price, was about $234 million. The estimated distribution of domestic primary antimony consumption was as follows: nonmetal products, including ceramics and glass and rubber products, 22%; flame retardants, 40%; and metal products, including antimonial lead and ammunition, 39%. Salient Statistics—United States: 2015 2016 2017 2018 2019e Production: Mine (recoverable antimony) — — — — — Smelter: Primary 645 664 621 331 370 Secondary 3,740 3,810 e3,800 e4,000 4,000 Imports for consumption: Ore and concentrates 308 119 61 96 140 Oxide 16,700 16,100 17,900 19,200 17,000 Unwrought, powder, waste and scrap1 5,790 7,150 6,830 6,500 7,200 Exports: Ore and concentrates1 31 -

Solid Solution in the As2s3-Sb2s3 Series at Zarshuran Gold Deposit, Iran
Archive of SID Journal of Sciences, Islamic Republic of Iran 19(2): 137-143 (2008) http://jsciences.ut.ac.ir University of Tehran, ISSN 1016-1104 Solid Solution in the As2S3-Sb2S3 Series at Zarshuran Gold Deposit, Iran B. Mehrabi* Department of Geology, Tarbiat Moalem University, 15614-Tehran, Islamic Republic of Iran Abstract Orpiment, getchellite and stibnite from Zarshuran deposit, NW Iran, exhibit extensive As-Sb substitution. There are four known minerals along As2S3-Sb2S3 join, i.e. orpiment (As2S3), Wakabayashilite [(As,Sb)20S30], getchellite (AsSbS3) and stibnite (Sb2S3). Extensive mineralogical studies by scanning electron microscope (SEM) and electron probe micro-analyzer (EPMA) indicate that getchellite from Zarshuran exhibits large As/Sb substitution, compared to the synthetic mineral and occurrences in other deposits, which justifies further experimental studies. Natural orpiment with the highest Sb content was recorded in Zarshuran samples. Considering the possible explanation for the incomplete record of solid solution, it appears that rapid variation in fluid chemistry and variability of As and Sb concentration in the hydrothermal solution may have caused extensive solid solution in the As2S3-Sb2S3 system. The recorded solid solution may indicate that temperatures of gold mineralization were less than 250°C which is supported by fluid inclusion data. Keywords: Getchellite; Orpiment; Stibnite; Solid solution; As2S3-Sb2S3 system; Zarshuran gold deposit sulphides, they are chemically analogous elements. The Introduction and Previous Work chemical analysis of most minerals in As-Sb-S systems Orpiment and stibnite have been recognized in indicates the presence of an incomplete solid solution several disseminated replacement type gold deposits in between orpiment and stibnite. -

C:\Documents and Settings\Alan Smithee\My Documents\MOTM
Itmd1//8Lhmdq`knesgdLnmsg9Rshamhsd This month’s mineral is among the most collectible of all minerals. Ours come from a Chinese antimony mine that is now recognized as a classic stibnite locality, and our write-up explains the unusual structure and properties of stibnite, describes its remarkable crystals, and details the many uses of antimony. OVERVIEW PHYSICAL PROPERTIES Chemistry: Sb2S3 Antimony Trisulfide, often containing some bismuth Class: Sulfides Group: Stibnite Crystal System: Orthorhombic Crystal Habits: Individual bladed or acicular prisms or jumbled aggregates of prisms, striated lengthwise; prisms often bent or twisted; also as radiated groups and granular and massive forms. Color: Lead-gray and gray-black to steel-gray and silvery-gray, sometimes with a bluish hue; occasionally iridescent; tarnish black. Luster: Metallic Transparency: Opaque Streak: Dark lead-gray Refractive Index: None (opaque) Cleavage: Perfect in one direction lengthwise Figure 1. Stibnite crytals. Fracture: Subconchoidal to irregular; brittle; thin prisms are slightly flexible. Hardness: 2.0 Specific Gravity: 4.6 Luminescence: None Distinctive Features and Tests: Best field identification features are softness; flexibility of thin prisms; and long, bladed and acicular crystal habits. Visually similar to bismuthinite [bismuth trisulfide, Bi2S3], which has considerably more density. Dana Classification Number: 2.11.2.1 NAME Stibnite, pronounced STIBB-nite, derives from stibi, the Greek name for the mineral. Stibnite has formerly been referred to as “antimonite,” “antimonide,” “sulfur antimonide,” “sulfur antimony,” “antimony glance,” “gray antimony,” “gray antimony ore,” “kohl,” “stibi,” “stimmi,” “stibium,” “lupus metallorum,” “platyophthalmite,” and “speissglas.” In current European mineralogical literature, stibnite appears as stibnit, stibnita, and stibnine. Antimony, pronounced ANN-tih-mow-nee, stems from the Latin word for the metal, antimonium, which means “opposed to solitude,” in allusion to its rare occurrence as a native element.