Antisense Oligonucleotide: Basic Concepts and Therapeutic Application in Inflammatory Bowel Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
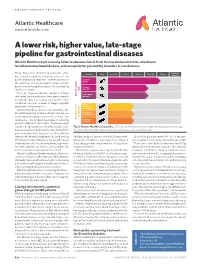
A Lower Risk, Higher Value, Late-Stage Pipeline for Gastrointestinal Diseases
ADVERTISEMENT FEATURE Atlantic Healthcare www.atlantichc.com A lower risk, higher value, late-stage pipeline for gastrointestinal diseases Atlantic Healthcare plc is raising funds to advance clinical trials for new chemical entities: alicaforsen for inflammatory bowel disease, and renzapride for gut motility disorders in rare diseases. Regulatory Many drugs miss clinical trial endpoints, often Indication Form Pre-clinical Phase 1 Phase 2a Phase 2b Phase 3 due to incorrect patient selection, choice of end approval points and dosing regimens. Further analysis of UC enema the data may reveal new insights and provide the Camligo basis for continuing development incorporating UC tablet AHC-1145 the lessons learnt. IBD There are many well-known examples of drugs Crohn’s tablet that failed for the indication they were originally Alicaforsen for AHC-1062 intended for, but successfully developed for other conditions; everyone is aware of Viagra, originally Systemic developed for hypertension. scleroderma GI complications Atlantic Healthcare, based near Cambridge, UK, Cystic fibrosis has established itself as a trans-Atlantic pharmaceu- GI complications tical company, building a diversified late-stage clini- GI Motility cal pipeline. The company specializes in acquiring Renzapride for Undisclosed indication products with robust clinical data. “We have brought together deep expertise to identify, acquire, com- Fig. 1 | Atlantic Healthcare’s pipeline. Offering risk diversification and targeting multiple patient groups. plete development, and market products directly to GI, gastrointestinal; IBD, inflammatory bowel disease; UC, ulcerative colitis. gastrointestinal (GI) clinicians,” said Toby Wilson Waterworth, Atlantic Healthcare’s CEO, and a Fellow William Sandborn, Director of the IBD Center at the GI motility disorders affect 10–15% of the gen- of the Royal Society of Medicine. -

Alicaforsen, an Antisense Inhibitor of ICAM-1, As Treatment for Left- Sided Ulcerative Colitis and Ulcerative Proctitis
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2017 Alicaforsen in the treatment of pouchitis Greuter, Thomas ; Rogler, Gerhard Abstract: Alicaforsen is a 20-base antisense oligonucleotide inhibiting ICAM-1 production, which is an important adhesion molecule involved in leukocyte migration and trafficking to the site of inflamma- tion. Hitherto, alicaforsen has been granted orphan drug designation and is prescribed as an unlicensed medicine in accordance with international regulation for the treatment of pouchitis and left-sided ulcer- ative colitis. Given the positive results evolving from one open-label trial and one case series in patients with chronic refractory pouchitis, US FDA has agreed to a rolling submission for a license application for the treatment of pouchitis, which has been recently initiated. Whether alicaforsen leads to higher endoscopic and clinical remission rates as placebo and whether the response can be maintained in the long-term in larger studies is yet unknown. An ongoing multicenter international Phase III trial will definitely address these unanswered questions. DOI: https://doi.org/10.2217/imt-2017-0085 Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-145563 Journal Article Accepted Version Originally published at: Greuter, Thomas; Rogler, Gerhard (2017). Alicaforsen in the treatment of pouchitis. Immunotherapy, 9(14):1143-1152. DOI: https://doi.org/10.2217/imt-2017-0085 -

Informed Consent
INFORMED CONSENT Exposing the Eugenics Cult: & Their Latest Experiment on Mankind: COVID-19 Written by: Umm Nadia, Taliah S. Muhammad Islamic Medicine Practitioner Divine Ayat Publications, LLC P.O. Box 29004 Henrico, Virginia 23242. Copyright © 2021 by Taliah S. Muhammad All rights reserved, including the right of reproduction in whole or in part in any form. ISBN 978-1-7370880-0-4 ISBN 978-1-7370880-1-1 (eBook) Table of Contents Introduction .................................................................................... 1 Always Speak The Truth ................................................................ 1 Informed Consent ........................................................................... 3 Chapter One ................................................................................ 16 Covid-19: The Plague That Strikes Behind The Ear .................... 16 Chapter Two ............................................................................... 45 What Our Hands Have Earned ..................................................... 45 Chapter Three ............................................................................. 64 The Makings Of A Cult ................................................................ 64 Chapter Four .............................................................................. 73 They Strove, They Achieved ........................................................ 73 Chapter Five .............................................................................. 113 Divine Knowledge In The Hands -

LAY SUMMARY CLINICAL RESEARCH PROTOCOL ACH UCP-301 Eudract Number 2013-002952-34 IND Number 64,905
LAY SUMMARY CLINICAL RESEARCH PROTOCOL ACH UCP-301 EudraCT Number 2013-002952-34 IND Number 64,905 Who Sponsored the Study? Atlantic Pharmaceuticals Ltd, Atlantic House, 10 Rose & Crown Walk, Saffron Walden, Essex, CB10 1JH, United Kingdom. Phone: +44 (0)1799 619 413 Email: [email protected] Website: www.atlantic.com Title of this Phase 3 Study: A Randomised, Double-Blind, Placebo-Controlled Trial of the Safety and Efficacy of Topical Alicaforsen Enema in Subjects with Active, Chronic, Antibiotic Refractory Primary Idiopathic Pouchitis. - In a Phase 3 study a new treatment is tested in many patients. - In this study, researchers compared the test medicine (alicaforsen enema) to placebo (identical looking enema liquid but with no medicine in it). - Randomising patients, by putting people (by chance) into 2 equal sized treatment groups, helps to reduce the differences between the groups, making the comparison between the groups fairer. - This trial was also “double-blinded”. This means that neither patients nor doctors knew who was given which treatment. This was done to make sure that the trial results were not influenced in any way. There was also an optional open-label-access follow-on-phase available to patients in this study (except for patients in France, for whom the national regulatory agency declined this part of the study). Alicaforsen enema is an investigational product designed to work in inflammatory bowel disease. In this study, the target was pouchitis. As the sponsor for this study, we at Atlantic Pharmaceuticals Ltd feel it is important for you to know the results. When the study ended, we created a scientific report of all the data. -

Alicaforsen for Pouchitis – Second Line
Horizon Scanning Centre July 2013 Alicaforsen for pouchitis – second line SUMMARY NIHR HSC ID: 4202 Alicaforsen is intended to be used as second line therapy for the treatment of pouchitis refractory to, or contra-indicated for, antibiotic therapy. If licensed, it will offer a treatment option for patients with pouchitis, for whom there are currently no approved effective therapies. Alicaforsen is administered by retention enema, and may also achieve topical drug delivery to the affected area while minimising systemic bioavailability. Alicaforsen is a first- This briefing is generation phosphorothioate-modified antisense oligodeoxynucleotide for the based on down regulation of intercellular adhesion molecule 1 (ICAM-1) messenger information RNA levels by RNase H-mediated degradation. Several studies have available at the time reported increased ICAM-1 expression within inflamed gut mucosa as well as of research and a increased serum concentrations of soluble ICAM-1 in pouchitis and limited literature experimental colitis. search. It is not intended to be a The prevalence of ulcerative colitis (UC) in the UK is estimated to be 243 per definitive statement 100,000 (equating to 136,264 people in England and Wales), with an annual on the safety, incidence of 10 per 100,000. Approximately 30% of patients with UC efficacy or eventually require colectomy, usually restorative proctocolectomy with ileal effectiveness of the pouch-anal anastomosis. Pouchitis is the most common long-term health technology complication after restorative proctocolectomy for UC, affecting up to 60% of covered and should patients and accounts for 10% of pouch failures. The quality of life for not be used for patients with pouchitis is adversely affected by their symptoms (e.g. -

Alicaforsen (ISIS 2302) Improves Clinical Symptoms of Pouchitis Patients
Alicaforsen (ISIS 2302) Improves Clinical Symptoms of Pouchitis Patients May 21, 2003 Results Support Antisense Drug Development Program in Inflammatory Bowel Disease ORLANDO, Fla., May 21 /PRNewswire-FirstCall/ -- Patients with pouchitis experience an improvement in clinical disease symptoms after receiving treatment with alicaforsen (ISIS 2302), an antisense inhibitor of the inflammatory target Intercellular Adhesion Molecule-1 (ICAM-1), according to results of an open-label, uncontrolled Phase II clinical trial. Improvements in endoscopic scores observed in the trial suggest there is long-term benefit of ICAM-1 inhibition in the treatment of pouchitis, an inflammatory bowel disease (IBD). Philip B. Miner, Jr., M.D., of The Oklahoma Foundation for Digestive Research, and principal investigator of the Phase II study, presented findings from the study yesterday at the 2003 Digestive Disease Week (DDW) meeting. Alicaforsen is being developed by Isis Pharmaceuticals, Inc., (Nasdaq: ISIS). The company has a broad development program for alicaforsen in IBD, including Phase III clinical trials in patients with Crohn's disease, and Phase II studies in patients with ulcerative colitis. The study was designed to evaluate both the efficacy and the safety of alicaforsen. The trial enrolled 12 patients with pouchitis. Patients received 240 mg of alicaforsen nightly for six weeks by enema administration, and will be followed for up to one year. Results from the first eight patients and one month of follow-up were reported at DDW. The primary endpoint of the trial was improvement in the Pouchitis Disease Activity Index (PDAI), a commonly used 18-point system that evaluates patients' symptom score, endoscopy and histology (each category is scored on a 0-6 scale). -

Clinical Trial of Alicaforsen for Patients with Antibiotic Refractory Pouchitis
Clinical trial of alicaforsen for patients with antibiotic refractory Pouchitis tlantic Pharmaceuticals Ltd is Each patient will receive a 6 week Aalerting pouchitis patients to a course of treatment – alicaforsen new study. Patient recruitment for the enema (240mg) to be self- study is now underway. administered at home, once nightly There is currently no licensed product (before going to bed). Response approved for the treatment of acute to treatment will be based on the flares of pouchitis, although the use of patient’s symptoms (collected by the antibiotics has become accepted as patient, daily, on an electronic diary the norm for many patients. card), which include reduction in relative stool frequency. Unfortunately, over a period of time the efficacy of antibiotics declines and Further assessment will be done patients may fail to respond – they through endoscopy data, with patients become “refractory” to treatment. requiring endoscopies at the start Alicaforsen enema is a new product of the trial, and at week 6, and 10. being investigated for the treatment of Patients will return to the clinic for “antibiotic refractory” pouchitis. safety and efficacy assessments at A Phase 3, randomised, double-blind, week 3, 6, 10, 18, 26. placebo-controlled parallel group Some patients will receive placebo in study will enrol adult patients in the trial, but this will be in addition approximately 40 sites across the U.S., to their standard medication. All Canada, Israel and Europe. patients will be eligible for open-label Alicaforsen works to reduce the levels alicaforsen post-trial, assuming of ICAM-1, a protein in the body which they meet appropriate is involved in inflammation. -

Isis Pharmaceuticals Initiates Phase II Clinical Trial of Alicaforsen, ISIS 2302, in Patients with Ulcerative Colitis
Isis Pharmaceuticals Initiates Phase II Clinical Trial of Alicaforsen, ISIS 2302, in Patients With Ulcerative Colitis November 21, 2002 CARLSBAD, Calif., Nov. 21 /PRNewswire-FirstCall/ -- Isis Pharmaceuticals, Inc. (Nasdaq: ISIS) announced today it has initiated a Phase II clinical trial of alicaforsen (ISIS 2302), an antisense inhibitor of intercellular adhesion molecule-1 (ICAM-1), in people with active ulcerative colitis (UC). The study will compare the safety and efficacy of an enema formulation of alicaforsen to mesalamine enema, a widely used topical medication for UC. An intravenous formulation of alicaforsen is being evaluated in a Phase III clinical program in people with Crohn's disease. "The early clinical data on the enema formulation of alicaforsen in ulcerative colitis are exciting. We are pleased to move this drug forward in development and to continue to expand the types of diseases that may be treated with this novel compound," said F. Andrew Dorr, M.D., Isis' Vice President and Chief Medical Officer. "Current treatment options for ulcerative colitis are limited, and we are optimistic that alicaforsen may be able to help address this critical medical need." The randomized, double-masked, active-controlled Phase II study is planned to enroll approximately 170 patients at 25 U.S. sites. Patients will be given alicaforsen enema or mesalamine enema once nightly for six weeks. The primary endpoint of the study is improvement in the Disease Activity Index (DAI) upon completion of the six-week dosing period and during a 12-month follow-up period. DAI is a common clinical index scoring system for the severity of symptoms related to UC. -

Alicaforsen for Active Ulcerative Colitis – Second Line
Horizon Scanning Research November 2015 & Intelligence Centre Alicaforsen for active ulcerative colitis – second line LAY SUMMARY Ulcerative colitis is a type of chronic inflammatory bowel disease that causes ulcers on the lining of the large bowel. Patients suffer with pain and diarrhoea and the condition may deteriorate and lead to hospital This briefing is admissions. Ulcerative colitis can have a large impact on patients’ based on quality of life. information available at the time Alicaforsen is a new treatment option that may reduce inflammation in of research and a the large bowel. It is taken once a day or once every other day as an limited literature enema. search. It is not intended to be a Alicaforsen is currently being studied to see how well it works and definitive statement on the safety, whether it is safe to use in people with ulcerative colitis. If alicaforsen efficacy or is licensed for use in the UK, it will offer a new treatment option for effectiveness of the people with active ulcerative colitis. health technology covered and should NIHR HSRIC ID: 672 not be used for commercial purposes or commissioning without additional information. This briefing presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health. NIHR Horizon Scanning Research & Intelligence Centre, University of Birmingham. Email: [email protected] Web: www.hsric.nihr.ac.uk Horizon Scanning Research & Intelligence Centre TARGET GROUP • Ulcerative colitis: distal colon; active – second line. -

A Survey of Commercial Biomolecules, Delimited to Pharmaceuticals and Medical Devices
TVE-K 17009 maj Examensarbete 15 hp 16 Juni 2017 A Survey of Commercial Biomolecules, Delimited to Pharmaceuticals and Medical Devices Markus Holowacz Annika Krans Camilla Wallén Alberto Martinez Nadia Mohammadi Abstract A Survey of Commercial Biomolecules, Delimited to Pharmaceuticals and Medical Devices Markus Holowacz, Annika Krans, Camilla Wallén, Alberto Martinez, Nadia Mohammadi Teknisk- naturvetenskaplig fakultet UTH-enheten Biologics — biomolecule-based therapeutics — are predicted to become the next generation of therapeutical drugs. The pharmaceutical industry is investing a great Besöksadress: amount of money into research in this medical field, which shows the importance and Ångströmlaboratoriet Lägerhyddsvägen 1 the need of analyses regarding biological drugs. This report is a survey of commercial Hus 4, Plan 0 biologics in which the market and trends regarding the past, present and future are analysed. The results are based on a literature study limited to commercial biologics Postadress: — either as chemical entities, as conjugates to another class of biomolecules or as Box 536 751 21 Uppsala medical devices. The market for biomolecule-based therapeutics and medical devices is predicted to flourish as the new generations of peptides, proteins, oligonucleotides, Telefon: carbohydrates and lipids increase in both approvals and sales. The fact that scientists 018 – 471 30 03 have gained a broader comprehension on a molecular level due to advanced Telefax: technologies has resulted in biologics reaching clinical maturity. The optimized 018 – 471 30 00 pharmacokinetic properties, greater specificity, and the increased therapeutic window have led to reduced side effects compared to traditional therapeutical alternatives. Hemsida: Due to new development approaches — peptidomimetics — the stability of peptides http://www.teknat.uu.se/student has shown to increase. -

Biological Therapies in Inflammatory Bowel Disease Beyond Anti-TNF Therapies
Clinical Immunology 206 (2019) 9–14 Contents lists available at ScienceDirect Clinical Immunology journal homepage: www.elsevier.com/locate/yclim Biological therapies in inflammatory bowel disease: Beyond anti-TNF therapies T Konstantinos H. Katsanosa,1, Konstantinos Papamichaelb,1, Joseph D. Feuersteinb, ⁎ Dimitrios K. Christodouloua, Adam S. Cheifetzb, a Division of Gastroenterology, University Hospital & Faculty of Medicine, School of Health Sciences, University of Ioannina, Greece b Center for Inflammatory Bowel Diseases, Division of Gastroenterology, Beth Israel Deaconess Medical Center, Harvard University, Boston, MA, USA ARTICLE INFO ABSTRACT Keywords: The pharmacological management of inflammatory bowel disease (IBD) over the last two decades has transi- Ulcerative colitis tioned from reliance on aminosalycilates, corticosteroids and immunomodulators to earlier treatment with anti- Crohn's disease tumor necrosis factor (anti-TNF) therapy. Nevertheless, 20–30% of patients discontinue anti-TNF therapy for Biologics primary non-response and another 30–40% for losing response within one year of treatment. These undesirable Novel therapies therapeutic outcomes can be attributed to pharmacokinetic (anti-drug antibodies and/or low drug concentra- S1P receptors tions) or pharmacodynamic issues characterized by a non-TNF driven inflammation. The latter issues necessitate Integrins ff Janus kinases the use of medications with di erent mechanisms of action. Besides the biologics natalizumab, vedolizumab and ustekinumab that have already been approved for the treatment of IBD new non-anti-TNF therapies are currently under investigation including small molecule drugs against Janus kinase and sphingosine-1-phosphate receptors. This manuscript will review the medications that are in the later stages of development for the treatment of IBD and directed against immune targets other than TNF. -

Clinical Trial of Alicaforsen for Patients with Antibiotic Refractory Pouchitis Atlantic Pharmaceuticals Ltd Is Alerting Pouchitis Patients to a New Study
Clinical trial of Alicaforsen for patients with antibiotic refractory Pouchitis Atlantic Pharmaceuticals Ltd is alerting pouchitis patients to a new study. Patient recruitment for the study is now underway. There is currently no licensed product approved for the treatment of acute flares of pouchitis, although the use of antibiotics has become accepted as the norm for many patients. Unfortunately, over a period of time the efficacy of antibiotics declines and patients may fail to respond – they become “refractory” to treatment. Alicaforsen enema is a new product being investigated for the treatment of “antibiotic refractory” pouchitis. A Phase 3, randomised, double- blind, placebo-controlled parallel group study will enrol adult patients in approximately 40 sites across the US, Canada, Israel and Europe. Alicaforsen works to reduce the levels of ICAM-1, a protein in the body which is involved in inflammation. Patients that are experiencing an acute flare of pouchitis will be eligible for the study (full selection criteria below). Each patient will receive a 6 week course of treatment – alicaforsen enema (240mg) to be self-ministered at home, once night (before going to bed). Response to treatment will be based on the patient’s symptoms (collected by the patient, daily, on an electronic diary card), which include reduction in relative stool frequency. Further assessment will be done through endoscopy data, with patients requiring endoscopies at the start of the trial, and at week 6, and 10. Patients will return to the clinic for safety and efficacy assessments at week, 3, 6, 10, 18, 26. Some patients will receive placebo in the trial, but this will be in addition to their standard medication.