The Atlas of Human African Trypanosomiasis: a Contribution To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PLAN DE RÉPONSE HUMANITAIRE République Centrafricaine
CYCLE DE PROGRAMMATION PLAN DE RÉPONSE HUMANITAIRE 2020 HUMANITAIRE PUBLIÉ EN DECEMBRE 2019 République Centrafricaine 1 PLAN DE REPONSE HUMANITAIRE 2020 | À PROPOS Pour consulter les mises à jour À propos les plus récentes : Ce document est consolidé par OCHA pour le compte de l’Équipe OCHA coordonne l’action humanitaire pour humanitaire pays et des partenaires humanitaires. Il présente les priorités garantir que les personnes affectées par une et les paramètres de la réponse stratégique de l’Équipe humanitaire crise reçoivent l’assistance et la protection dont elles ont besoin. OCHA s’efforce pays, basés sur une compréhension partagée de la crise, énoncés dans de surmonter les obstacles empêchant l’Aperçu des besoins humanitaires. l’assistance humanitaire de joindre les Les désignations employées et la présentation des éléments dans le personnes affectées par des crises et est chef présent rapport ne signifient pas l’expression d’une quelque opinion que de file dans la mobilisation de l’assistance et de ressources pour le compte du système ce soit de la Partie du Secrétariat des Nations unies concernant le statut humanitaire juridique d’un pays, d’un territoire, d’une ville ou d’une zone ou de leurs autorités ou concernant la délimitation de frontières ou de limites. www.unocha.org/car twitter: @OCHA_CAR PHOTO DE COUVERTURE @UNICEF CAR / B. Matous Humanitarian Réponse est destiné à être le site Web central des outils et des services de Gestion de l’information permettant l’échange d’informations entre les clusters et les membres du IASC intervenant dans une crise. car.humanitarianresponse.info Humanitarian InSight aide les décideurs en leur donnant accès à des données humanitaires essentielles. -

472,864 25% USD 243.8 Million
CENTRAL AFRICAN REPUBLIC SITUATION UNHCR REGIONAL UPDATE 65 1 – 29 February 2016 HIGHLIGHTS KEY FIGURES . In the Central African Republic, UNHCR finalized the IDP registration 472,864 exercise in the capital city Bangui and provided emergency assistance to Central African refugees in displaced families affected by multiple fire outbreaks in IDP sites; Cameroon, Chad, DRC and Congo . UNHCR launched a youth community project in Chad, an initiative providing young Central African refugees with the opportunity to develop their own projects; . The biometric registration of urban refugees in Cameroon is completed in the 25% capital Yaoundé and ongoing in the city of Douala; IDPs in CAR located in the capital . In the Democratic Republic of the Congo, UNHCR registered 4,376 Central Bangui African refugees living on several islands in the Ubangi River; . Refugees benefit from a country-wide vaccination campaign in the Republic of the Congo. The persistent dire funding situation of UNHCR’s operations in all countries is worrisome and additional contributions are required immediately to meet FUNDING urgent protection and humanitarian needs. USD 243.8 million required for the situation in 2016 917,131 persons of concern Funded 0.2% IDPs in CAR 435,165 Gap Refugees in 270,562 99.8% Cameroon Refugees in DRC 108,107 PRIORITIES Refugees in Chad 65,961 . CAR: Reinforce protection mechanisms from sexual exploitation and abuse and strengthen coordination with Refugees in Congo 28,234 relevant actors. Cameroon: Continue biometric registration; strengthen the WASH response in all refugee sites. Chad: Strengthen advocacy to improve refugees’ access to arable land; promote refugee’s self-sufficiency. -

Central African Republic Emergency Situation UNHCR Regional Bureau for Africa As of 26 September 2014
Central African Republic Emergency Situation UNHCR Regional Bureau for Africa as of 26 September 2014 N'Djamena UNHCR Representation NIGERIA UNHCR Sub-Office Kerfi SUDAN UNHCR Field Office Bir Nahal Maroua UNHCR Field Unit CHAD Refugee Sites 18,000 Haraze Town/Village of interest Birao Instability area Moyo VAKAGA CAR refugees since 1 Dec 2013 Sarh Number of IDPs Moundou Doba Entry points Belom Ndele Dosseye Sam Ouandja Amboko Sido Maro Gondje Moyen Sido BAMINGUI- Goré Kabo Bitoye BANGORAN Bekoninga NANA- Yamba Markounda Batangafo HAUTE-KOTTO Borgop Bocaranga GRIBIZI Paoua OUHAM 487,580 Ngam CAMEROON OUHAM Nana Bakassa Kaga Bandoro Ngaoui SOUTH SUDAN Meiganga PENDÉ Gbatoua Ngodole Bouca OUAKA Bozoum Bossangoa Total population Garoua Boulai Bambari HAUT- Sibut of CAR refugees Bouar MBOMOU GadoNANA- Grimari Cameroon 236,685 Betare Oya Yaloké Bossembélé MBOMOU MAMBÉRÉ KÉMO Zemio Chad 95,326 Damara DR Congo 66,881 Carnot Boali BASSE- Bertoua Timangolo Gbiti MAMBÉRÉ- OMBELLA Congo 19,556 LOBAYE Bangui KOTTO KADÉÏ M'POKO Mbobayi Total 418,448 Batouri Lolo Kentzou Berbérati Boda Zongo Ango Mbilé Yaoundé Gamboula Mbaiki Mole Gbadolite Gari Gombo Inke Yakoma Mboti Yokadouma Boyabu Nola Batalimo 130,200 Libenge 62,580 IDPs Mboy in Bangui SANGHA- Enyelle 22,214 MBAÉRÉ Betou Creation date: 26 Sep 2014 Batanga Sources: UNCS, SIGCAF, UNHCR 9,664 Feedback: [email protected] Impfondo Filename: caf_reference_131216 DEMOCRATIC REPUBLIC The boundaries and names shown and the OF THE CONGO designations used on this map do not imply GABON official endorsement or acceptance by the United CONGO Nations. Final boundary between the Republic of Sudan and the Republic of South Sudan has not yet been determined. -

Central African Republic Humanitarian Situation Report
Central African Republic Humanitarian Situation Report © UNICEFCAR/2018/Jonnaert September 2018 SITUATION IN NUMBERS Highlights 1.3 million # of children in need of humanitarian assistance - On 17 September, the school year was officially launched by the President in Bangui. UNICEF technically and financially supported 2.5 million the Ministry of Education (MoE) in the implementation of the # of people in need (OCHA, June 2018) national ‘Back to School’ mass communication campaign in all 8 Academic Inspections. The Education Cluster estimates that 280,000 621,035 school-age children were displaced, including 116,000 who had # of Internally displaced persons (OCHA, August 2018) dropped out of school Outside CAR - The Rapid Response Mechanism (RRM) hit a record month, with partners ensuring 10 interventions across crisis-affected areas, 572, 984 reaching 38,640 children and family members with NFI kits, and # of registered CAR refugees 59,443 with WASH services (UNHCR, August 2018) - In September, 19 violent incidents against humanitarian actors were 2018 UNICEF Appeal recorded, including UNICEF partners, leading to interruptions of assistance, just as dozens of thousands of new IDPs fleeing violence US$ 56.5 million reached Bria Sector/Cluster UNICEF Funding status* (US$) Key Programme Indicators Cluster Cumulative UNICEF Cumulative Target results (#) Target results (#) WASH: Number of affected people Funding gap : Funds provided with access to improved 900,000 633,795 600,000 82,140 $32.4M (57%) received: sources of water as per agreed $24.6M standards Education: Number of Children (boys and girls 3-17yrs) in areas 94,400 79,741 85,000 69,719 affected by crisis accessing education Required: Health: Number of children under 5 $56.5M in IDP sites and enclaves with access N/A 500,000 13,053 to essential health services and medicines. -

Interagency Assessment Report
AA SS SS EE SS SS MM EE NN TT RR EE PP OO RR TT Clearing of site for returning refugees from Chad, Moyenne Sido, 18 Feb. 2008 Moyenne Sido Sous-Prefecture, Ouham 17 – 19 February 2008 Contents 1. Introduction & Context………………………………………………………………. 2. Participants & Itinerary ……………………………………………………………... 3. Key Findings …………………………………………………. ……………………. 4. Assessment Methodology………………………………………………………..... 5. Sectoral Findings: NFI’s, Health & Nutrition, WASH, Protection, Education ………………………. 6. Recommendations & Proposed Response…………. …………….................... 2 Introduction & Context The sous prefecture of Moyenne Sido came into creation in March 2007, making it the newest sous- prefecture in the country. As a result, it has a little civil structure and has not received any major investment from the governmental level, and currently there is only the Mayor who is struggling to meet the needs of both the returnees and the host population, the latter is estimated at 17,000 persons. During the course of the visit, he was followed by several groups of elderly men (heads of families) who were requesting food from him, or any other form of assistance. The Mayor was distressed that neither the governmental authorities nor humanitarian agencies have yet been able to provide him with advice or assistance. The returnees are mainly Central African Republic citizens who fled to southern Chad after the coup in 2003. Since then, they have been residing at Yarounga refugee camp in Chad. Nine months ago, they received seeds from an NGO called Africa Concern, which they were supposed to plant and therefore be able to provide food for themselves. However, the returnees have consistently stated that the land they were set aside for farming was dry and arid, and they were unable to grow their crops there. -

The Central African Republic Diamond Database—A Geodatabase of Archival Diamond Occurrences and Areas of Recent Artisanal and Small-Scale Diamond Mining
Prepared in cooperation with the U.S. Agency for International Development under the auspices of the U.S. Department of State The Central African Republic Diamond Database—A Geodatabase of Archival Diamond Occurrences and Areas of Recent Artisanal and Small-Scale Diamond Mining Open-File Report 2018–1088 U.S. Department of the Interior U.S. Geological Survey Cover. The main road west of Bambari toward Bria and the Mouka-Ouadda plateau, Central African Republic, 2006. Photograph by Peter Chirico, U.S. Geological Survey. The Central African Republic Diamond Database—A Geodatabase of Archival Diamond Occurrences and Areas of Recent Artisanal and Small-Scale Diamond Mining By Jessica D. DeWitt, Peter G. Chirico, Sarah E. Bergstresser, and Inga E. Clark Prepared in cooperation with the U.S. Agency for International Development under the auspices of the U.S. Department of State Open-File Report 2018–1088 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior RYAN K. ZINKE, Secretary U.S. Geological Survey James F. Reilly II, Director U.S. Geological Survey, Reston, Virginia: 2018 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment—visit https://www.usgs.gov or call 1–888–ASK–USGS. For an overview of USGS information products, including maps, imagery, and publications, visit https://store.usgs.gov. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this information product, for the most part, is in the public domain, it also may contain copyrighted materials as noted in the text. -

MSF: Unprotected: Summary of Internal Review on the October 31St Events in Batangafo, Central African Republic
MSF February 2019 Unprotected Summary of internal review on the October 31st events in Batangafo, Central African Republic Unprotected. Summary of internal review on the October 31st events in Batangafo, Central African Republic. February 2019 The Centre for Applied Reflection on Humanitarian Practice (ARHP) documents and reflects upon the operational challenges and dilemmas faced by the field teams of the MSF Operational Centre Barcelona (MSF OCBA). This report is available at the ARHP website: https://arhp.msf.es © MSF C/ Nou de la Rambla, 26 08001 Barcelona Spain Pictures by Helena Cardellach. FRONT-PAGE PICTURE: IDP site burned, November 1st 2018. Batangafo, Central African Republic. Contents 5 Methodology 7 Executive summary 9 Brief chronology of events 10 Violence-related background of Central African Republic and Batangafo 15 Description and analysis of events 20 Consequences of violence 23 The humanitarian response 25 A hospital at the centre of the power struggle 28 Civilians UN-protected? 29 Conclusions 31 Asks 32 MSF in CAR and Batangafo 3 MSF | Unprotected. Summary of internal review on the October 31st events in Batangafo (CAR) Acronyms used AB Antibalaka CAR Central African Republic DRC Danish Refugee Council ES Ex Séléka FPRC Popular Front for the Rebirth of CAR GoCAR Government of CAR HC Humanitarian Coordinator (UN) IDP Internal Displaced People IHL International Humanitarian Law MINUSCA UN Multidimensional Integrated Stabilization Mission in CAR MPC Central African Patriotic Movement TOB (UN) Office for the Coordination of Humanitarian Affairs UN Temporary Operational Base UNOCHA United Nations UNSC UN Security Council 4 MSF | Unprotected. Summary of internal review on the October 31st events in Batangafo (CAR) Methodology This report has been elaborated by the MSF’s Centre for Applied Research on Humanitarian Practice (ARHP) between November 2018 and early January 2019. -

Central African Rep.: Sub-Prefectures 09 Jun 2015
Central African Rep.: Sub-Prefectures 09 Jun 2015 NIGERIA Maroua SUDAN Birao Birao Abyei REP. OF Garoua CHAD Ouanda-Djallé Ouanda-Djalle Ndélé Ndele Ouadda Ouadda Kabo Bamingui SOUTH Markounda Kabo Ngaounday Bamingui SUDAN Markounda CAMEROON Djakon Mbodo Dompta Batangafo Yalinga Goundjel Ndip Ngaoundaye Boguila Batangafo Belel Yamba Paoua Nangha Kaga-Bandoro Digou Bocaranga Nana-Bakassa Borgop Yarmbang Boguila Mbrès Nyambaka Adamou Djohong Ouro-Adde Koui Nana-Bakassa Kaga-Bandoro Dakere Babongo Ngaoui Koui Mboula Mbarang Fada Djohong Garga Pela Bocaranga MbrÞs Bria Djéma Ngam Bigoro Garga Bria Meiganga Alhamdou Bouca Bakala Ippy Yalinga Simi Libona Ngazi Meidougou Bagodo Bozoum Dekoa Goro Ippy Dir Kounde Gadi Lokoti Bozoum Bouca Gbatoua Gbatoua Bakala Foulbe Dékoa Godole Mala Mbale Bossangoa Djema Bindiba Dang Mbonga Bouar Gado Bossemtélé Rafai Patou Garoua-BoulaiBadzere Baboua Bouar Mborguene Baoro Sibut Grimari Bambari Bakouma Yokosire Baboua Bossemptele Sibut Grimari Betare Mombal Bogangolo Bambari Ndokayo Nandoungue Yaloké Bakouma Oya Zémio Sodenou Zembe Baoro Bogangolo Obo Bambouti Ndanga Abba Yaloke Obo Borongo Bossembele Ndjoukou Bambouti Woumbou Mingala Gandima Garga Abba Bossembélé Djoukou Guiwa Sarali Ouli Tocktoyo Mingala Kouango Alindao Yangamo Carnot Damara Kouango Bangassou Rafa´ Zemio Zémio Samba Kette Gadzi Boali Damara Alindao Roma Carnot Boulembe Mboumama Bedobo Amada-Gaza Gadzi Bangassou Adinkol Boubara Amada-Gaza Boganangone Boali Gambo Mandjou Boganangone Kembe Gbakim Gamboula Zangba Gambo Belebina Bombe Kembé Ouango -
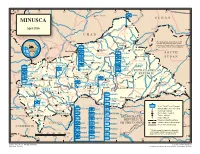
MINUSCA T a Ou M L B U a a O L H R a R S H Birao E a L April 2016 R B Al Fifi 'A 10 H R 10 ° a a ° B B C H a VAKAGA R I CHAD
14° 16° 18° 20° 22° 24° 26° ZAMBIA Am Timan é Aoukal SUDAN MINUSCA t a ou m l B u a a O l h a r r S h Birao e a l April 2016 r B Al Fifi 'A 10 h r 10 ° a a ° B b C h a VAKAGA r i CHAD Sarh Garba The boundaries and names shown ouk ahr A Ouanda and the designations used on this B Djallé map do not imply official endorsement Doba HQ Sector Center or acceptance by the United Nations. CENTRAL AFRICAN Sam Ouandja Ndélé K REPUBLIC Maïkouma PAKISTAN o t t SOUTH BAMINGUI HQ Sector East o BANGORAN 8 BANGLADESH Kaouadja 8° ° SUDAN Goré i MOROCCO u a g n i n i Kabo n BANGLADESH i V i u HAUTE-KOTTO b b g BENIN i Markounda i Bamingui n r r i Sector G Batangafo G PAKISTAN m Paoua a CAMBODIA HQ Sector West B EAST CAMEROON Kaga Bandoro Yangalia RWANDA CENTRAL AFRICAN BANGLADESH m a NANA Mbrès h OUAKA REPUBLIC OUHAM u GRÉBIZI HAUT- O ka Bria Yalinga Bossangoa o NIGER -PENDÉ a k MBOMOU Bouca u n Dékoa MAURITANIA i O h Bozoum C FPU CAMEROON 1 OUHAM Ippy i 6 BURUNDI Sector r Djéma 6 ° a ° Bambari b ra Bouar CENTER M Ouar Baoro Sector Sibut Baboua Grimari Bakouma NANA-MAMBÉRÉ KÉMO- BASSE MBOMOU M WEST Obo a Yaloke KOTTO m Bossembélé GRIBINGUI M b angúi bo er ub FPU BURUNDI 1 mo e OMBELLA-MPOKOYaloke Zémio u O Rafaï Boali Kouango Carnot L Bangassou o FPU BURUNDI 2 MAMBÉRÉ b a y -KADEI CONGO e Bangui Boda FPU CAMEROON 2 Berberati Ouango JTB Joint Task Force Bangui LOBAYE i Gamboula FORCE HQ FPU CONGO Miltary Observer Position 4 Kade HQ EGYPT 4° ° Mbaïki Uele National Capital SANGHA Bondo Mongoumba JTB INDONESIA FPU MAURITANIA Préfecture Capital Yokadouma Tomori Nola Town, Village DEMOCRATICDEMOCRATIC Major Airport MBAÉRÉ UNPOL PAKISTAN PSU RWANDA REPUBLICREPUBLIC International Boundary Salo i Titule g Undetermined Boundary* CONGO n EGYPT PERU OFOF THE THE CONGO CONGO a FPU RWANDA 1 a Préfecture Boundary h b g CAMEROON U Buta n GABON SENEGAL a gala FPU RWANDA 2 S n o M * Final boundary between the Republic RWANDA SERBIA Bumba of the Sudan and the Republic of South 0 50 100 150 200 250 km FPU SENEGAL Sudan has not yet been determined. -

CMP Juin 2020 STATISTIQUES DETAILLEES DES SITES Pdis EN
Legende CMP Juin 2020 Type-site: En hausse Update S= site Stable STATISTIQUES DETAILLEES DES SITES PDIs EN RCA L= Lieu de Regroupement En baisse No Update Differe # Préfecture Sous-Préfecture Commune Localité Site Type-site 31.05.2020 30.06.2020 Evolution Date Update Update Commentaires Sources de Donnees nce 1 Haut-Mbomou Zemio Zemio Zemio Site D L 2771 2853 3% juin-20 Profilage DTM IOM 205 PDIs venant de l'axe Bambouti suite aux exactions des 2 Haut-Mbomou Obo Obo Obo Gougbere S 2410 2410 0% Novembre 2019 ASA elements armes assimiles a l'UPC 3 Haut-Mbomou Obo Obo Obo Ligoua S 1180 1180 0% Juin 2019 COOPI 4 Haut-Mbomou Obo Obo Obo Nguilinguili S 455 455 0% Juin 2019 COOPI 5 Haut-Mbomou Obo Obo Obo Zemio S 693 693 0% Mars 2020 Profilage DTM IOM 6 Haut-Mbomou Obo Obo Mboki Mboki S 1579 2889 83% juin-20 Des PDIs venus de Obo entre Mai et juin Mission InterAgence 7 Haut-Mbomou Obo Obo Obo Catolique L 300 400 33% juin-20 Informateur clef le Prete de l'Eglise Catholique CCCM 8 Haut-Mbomou Djemah Djemah Kadjima A1 L 610 610 0% Mars 2020 Profilage DTM IOM 9 Haut-Mbomou Djemah Djemah Kadjima A2 L 625 625 0% Mars 2020 Profilage DTM IOM 10 Haut-Mbomou Djemah Djemah Kadjima B1 L 640 640 0% Mars 2020 Profilage DTM IOM 11 Haut-Mbomou Djemah Djemah Kadjima B2 L 675 675 0% Mars 2020 Profilage DTM IOM 12 Haut-Mbomou Djemah Djemah Kadjima C1 L 1085 1085 0% Mars 2020 Profilage DTM IOM Site catholique Petit ASA-Profilage 13 Mbomou Bangassou Bangassou Bangassou S 2012 2052 2% juin-20 ASA Seminaire 14 Mbomou Rafaï Rafaï AIM Site Aim S 185 185 0% Mai 2020 -

République Centrafricaine - Préfecture : Kémo Date De Production :Février 2015
Pour Usage Humanitaire Uniquement République Centrafricaine - Préfecture : Kémo Date de production :février 2015 Bazoyua Mbrés Bongo Mbrès Tao Wangué 1 Bobani Karagoua Bonou 2 Lady Lakouéténé Zimi-Gbagoua Zamboutou Gbawi Bokoga Yangoumara Gbada-Wangue Ndjangala Ouham Botto Fafa Gokara Boua Bambia 1 Mbiti Mbrès Badolé Bambia 2 Ndenga Kanda Nana-Gribizi Mbrés Sabayanga 2 Boboin Sabayanga Kaga-Bandoro Scieurs Bogoué Boya Gribizi 1 Bokolo Bogoué Bokago Somboké Morobanda 1 Yandoba Morobanda 2 Bokaga Beya Mbambi Bouca Bayolo Gboréa Bérabofidoua Banou Togbo Bac Bongoyo 1 Koumi Mboussa Mbouilli Mbolokpaka Baguiti 2 Begbayolo Bouloua Béboguila Koua Dissikou 4 Dissikou 3 Wapo Banda-Mandja 2 Dissikou Bofoulou Béra-Bobo Bokada Baguiti 1 Ba-Bobo Orongou 2 Orongou 1 Dekoa Bozagba Bouca Bofere Wandalongo Bobo Mbou Gou 2 Gou 1 Bombaroua Gbegon Begueze Yaligaza Daya Kagaya Bégou Bofidoua 2 Bafada Boanga Yangassa Bandagoli Baguela Kobadja Baïdou-Ngoumbourou Sidi-Ndolo Bakala Banda-Mandja 1 Lah Dekoa Saboyombo Ouolo 1 Plémandji Bengbali Begbaranga Malékara Ippy Oualo Ngbéré Tilo Koudou-Bégo Gpt Bobatoua 2 Niamou Tilo Binguifara Bedonga Gpt Donzi Yombandji Bekofe 1 Gazaporo Bekofe 2 Ngoro Bédambou Zourou Bovoula Baguiti 2 Mbimbi Fôh Cotonaf Tilo Simandele Tilo Madomalé Pélékesse Guiffa Ndéré Bodo Bongo 2 Bokoro Zouhouli Bongo Danga-Gboudou Dékoa Badéré Poukouya Bambari Sabone Koudoukou Oualambélé Mourouba Ngarambéti Mbimé-Yomba Bodengue Mbadjié Dobalé Ndakadja Ouham Bouca Mala Yonga Mabanga Bakabi Katakpa Mala Ndamiri Yomba Bakala Binguimalé Piangou Oumari -

What Drives Mortality Among HIV Patients in a Conflict Setting?
Crellen et al. Conflict and Health (2019) 13:52 https://doi.org/10.1186/s13031-019-0236-7 RESEARCH Open Access What drives mortality among HIV patients in a conflict setting? A prospective cohort study in the Central African Republic Thomas Crellen1,2* , Charles Ssonko3,TuridPiening4, Marcel Mbeko Simaleko5, Karen Geiger1 and M. Ruby Siddiqui3 Abstract Background: Provision of antiretroviral therapy (ART) in conflict settings is rarely attempted and little is known about the expected patterns of mortality. The Central African Republic (CAR) continues to have a low coverage of ART despite an estimated 110,000 people living with HIV and 5000 AIDS-related deaths in 2018. We present results from a cohort in Zemio, Haut-Mboumou prefecture. This region had the highest prevalence of HIV nationally (14.8% in a 2010 survey), and was subject to repeated attacks by armed groups on civilians during the observed period. Methods: Conflict from armed groups can impact cohort mortality rates i) directly if HIV patients are victims of armed conflict, or ii) indirectly if population displacement or fear of movement reduces access to ART. Using monthly counts of civilian deaths, injuries and abductions, we estimated the impact of the conflict on patient mortality. We also determined patient-level risk factors for mortality and how the risk of mortality varies with time spent in the cohort. Model-fitting was performed in a Bayesian framework, using logistic regression with terms accounting for temporal autocorrelation. Results: Patients were recruited and observed in the HIV treatment program from October 2011 to May 2017. Overall 1631 patients were enrolled and 1628 were included in the analysis giving 48,430 person-months at risk and 145 deaths.