Recovery Plans for Powelliphanta Land Snails, 2003-2013
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Schedule D Part3
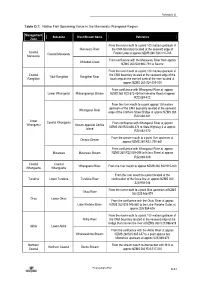
Schedule D Table D.7: Native Fish Spawning Value in the Manawatu-Wanganui Region Management Sub-zone River/Stream Name Reference Zone From the river mouth to a point 100 metres upstream of Manawatu River the CMA boundary located at the seaward edge of Coastal Coastal Manawatu Foxton Loop at approx NZMS 260 S24:010-765 Manawatu From confluence with the Manawatu River from approx Whitebait Creek NZMS 260 S24:982-791 to Source From the river mouth to a point 100 metres upstream of Coastal the CMA boundary located at the seaward edge of the Tidal Rangitikei Rangitikei River Rangitikei boat ramp on the true left bank of the river located at approx NZMS 260 S24:009-000 From confluence with Whanganui River at approx Lower Whanganui Mateongaonga Stream NZMS 260 R22:873-434 to Kaimatira Road at approx R22:889-422 From the river mouth to a point approx 100 metres upstream of the CMA boundary located at the seaward Whanganui River edge of the Cobham Street Bridge at approx NZMS 260 R22:848-381 Lower Coastal Whanganui From confluence with Whanganui River at approx Whanganui Stream opposite Corliss NZMS 260 R22:836-374 to State Highway 3 at approx Island R22:862-370 From the stream mouth to a point 1km upstream at Omapu Stream approx NZMS 260 R22: 750-441 From confluence with Whanganui River at approx Matarawa Matarawa Stream NZMS 260 R22:858-398 to Ikitara Street at approx R22:869-409 Coastal Coastal Whangaehu River From the river mouth to approx NZMS 260 S22:915-300 Whangaehu Whangaehu From the river mouth to a point located at the Turakina Lower -

Classifications
Classifications rt.code.desc Classifications Code Classifications rt.code.base Akitio River Scheme - River Maintenance RC Direct Benefit AREA Akitio River Scheme - Contributor CN Contributor AREA Ashhurst Scheme - Flood Protection AC Flooding Urban CAPITAL Ashhurst Scheme - Flood Protection SUIP AN Annual Charge TARGET Ashhurst Scheme - Lower Stream Maintenance AL Channel Maintenance High AREA Ashhurst Scheme - Upper Stream Maintenance AU Channel Maintenance Low AREA Eastern Manawatu - Lower River Maintenance EL Channell Maintenane High AREA Eastern Manawatu - Upper River Maintenance EU Channell Maintenance low AREA Eastern Manawatu River Scheme - Contributor CN Contributor AREA Eastern Manawatu River Scheme - Indirect IN Indirect Benefit TARGET Forest Road Drainage Scheme A High Benefit AREA Forest Road Drainage Scheme B Medium Benefit AREA Forest Road Drainage Scheme C Moderate Benefit AREA Forest Road Drainage Scheme D Low Benefit AREA Forest Road Drainage Scheme E Minor Benefit AREA Forest Road Drainage Scheme F Indirect Benefit AREA Foxton East Drainage Scheme D1 High Benefit AREA Foxton East Drainage Scheme D2 Medium Benefit AREA Foxton East Drainage Scheme D3 Moderate Benefit AREA Foxton East Drainage Scheme D4 Minor Benefit AREA Foxton East Drainage Scheme D5 Low Benefit AREA Foxton East Drainage Scheme SUIP AC Annual Charge TARGET Foxton East Drainage Scheme Urban U1 Urban CAPITAL Haunui Drainage Scheme A Direct Benefit CAPITAL Himatangi Drainage Scheme A High Benefit AREA Himatangi Drainage Scheme B Medium Benefit AREA Himatangi -

Feilding Manawatu Palmerston North City
Mangaweka Adventure Company (G1) Rangiwahia Scenic Reserve (H2) Location: 143 Ruahine Road, Mangaweka. Phone: +64 6 382 5744 (See Manawatu Scenic Route) OFFICIAL VISITOR GUIDE OFFICIAL VISITOR GUIDE Website: www.mangaweka.co.nz The best way to experience the mighty Rangitikei River is with these guys. Guided kayaking and rafting Robotic Dairy Farm Manawatu(F6) trips for all abilities are on offer, and the friendly crew will make sure you have an awesome time. Location: Bunnythorpe. Phone: +64 27 632 7451 Bookings preferred but not essential. Located less than 1km off State Highway 1! Website: www.robotfarmnz.wixsite.com/robotfarmnz Take a farm tour and watch the clever cows milk themselves in the amazing robotic milking machines, Mangaweka Campgrounds (G1) experience biological, pasture-based, free-range, sustainable, robotic farming. Bookings are essential. Location: 118 Ruahine Road, Mangaweka. Phone: +64 6 382 5744 Website: www.mangaweka.co.nz An idyllic spot for a fun Kiwi camp experience. There are lots of options available from here including The Coach House Museum (E5) rafting, kayaking, fishing, camping or just relaxing under the native trees. You can hire a cabin that Location: 121 South Street, Feilding. Phone: +64 6 323 6401 includes a full kitchen, private fire pit and wood-burning barbecue. Website: www.coachhousemuseum.org Discover the romance, hardships, innovation and spirit of the early Feilding and Manawatu pioneers Mangaweka Gallery and Homestay (G1) through their stories, photos and the various transportation methods they used, all on display in an Location: The Yellow Church, State Highway 1, Mangaweka. Phone: +64 6 382 5774 outstanding collection of rural New Zealand heritage, showcasing over 140 years of history. -

A Record of Natural and Human-Induced Environmental
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. A record of natural and human- induced environmental change from Lake Horowhenua A thesis presented in partial fulfilment of the requirements for the degree of Master of Science in Earth Science School of Agriculture and Natural Environment, Massey University, Palmerston North, New Zealand Celeste Bevins 2019 Lake Horowhenua at sunset. Abstract Lake Horowhenua is a hypertrophic turbid lake located in the western coastal plain of the lower North Island of New Zealand. In order to effectively restore or manage modified systems such as Lake Horowhenua, an understanding of past environmental change and natural variability is essential to provide a benchmark for ‘natural’ conditions. Cores from the bed of Lake Horowhenua have been analysed to reconstruct a detailed environmental record for the last c. 4,200 cal yr BP. Prior to lake formation, the area now occupied by the lake was subject to fluvial deposition from the Ohau River sometime prior to 7,500 cal yr BP. Dune transgression began in the region c. 7,700 cal yr BP at the very earliest, and drainage of the small streams and springs was impeded, allowing for the formation of a proto lake. A tidal surge up the Hokio Stream may have occurred c. 7,100 cal yr BP. Clastic delivery into the lake from the inflowing streams was high from 4,200 cal yr BP until sometime around 3,200 cal yr BP. -

Manawatu-Whanganui Regional Sports Facility Plan Is to Provide a High Level Strategic Framework for Sport and Recreation Facility Planning Across the Region (Map 1)
MANAWATU - WHANGANUI REGIONAL SPORT FACILITY PLAN REFERENCE REPORT MARCH 2018 Foreword – Sport New Zealand Sport New Zealand aims to get more young people and adults into sport and active recreation and produce more winners on the worlds sporting stage. It does this through its strategic approach for Community Sport and High Performance Sport outcomes. Spaces, places, and facilities for sport is one of five strategic priorities in the Community Sport Strategy with a goal to develop and sustain a world leading community sport system where the need of the participant and athlete is the focus. With leadership from the network of Regional Sports Trusts, Sport NZ is actively supporting better decision making and investment for future sporting spaces and places through a collaborative regional approach with local and regional government, education, Iwi, funders, national and regional sports organisations. The drivers for taking a regional approach to facility planning can be one or more of the following: • The desire of funders to invest wisely in identified priority projects that will make the most impact • An ageing network of facilities needing refurbishment, re-purposing, replacement or removal • Changing demographics within a community, such as an increase in the population. • Changing participation trends nationally and within a region requiring new types of facilities, or a new use of an existing facility • Increasing expectations of users and user groups • A growing acknowledgement that there is a hierarchy of facilities – regional, sub-regional and local – and that regional collaboration is the only fair and reasonable way to build and manage regional and sub-regional facilities. -

Agenda of Regional Transport Committee
I hereby give notice that an ordinary meeting of the Regional Transport Committee will be held on: Date: Tuesday, 3 September 2019 Time: 10.30am Venue: Tararua Room Horizons Regional Council 11-15 Victoria Avenue, Palmerston North REGIONAL TRANSPORT COMMITTEE AGENDA MEMBERSHIP Chair Cr EB Gordon JP Horizons Regional Council Cr RJ Keedwell Horizons Regional Council Mayor M Feyen Horowhenua District Council Mayor H Worboys Manawatu District Council Ms E Speight New Zealand Transport Agency Mayor G Smith Palmerston North City Council Mayor A Watson Rangitikei District Council Mayor D Cameron Ruapehu District Council Mayor T Collis Tararua District Council Mayor H McDouall Whanganui District Council Advisory Mr E Christiansen Road Users Representative New Zealand Police Mr Sandy Walker Road Transport Association Mr Anthony Mills KiwiRail Ms S Lampkin Active Transport/Public Transport Michael McCartney Chief Executive Contact Telephone: 0508 800 800 Email: [email protected] Postal Address: Private Bag 11025, Palmerston North 4442 Full Agendas are available on Horizons Regional Council website www.horizons.govt.nz Note: The reports contained within this agenda are for consideration and should not be construed as Council policy unless and until adopted. Items in the agenda may be subject to amendment or withdrawal at the meeting. for further information regarding this agenda, please contact: Julie Kennedy, 06 9522 800 CONTACTS 24 hr Freephone : [email protected] www.horizons.govt.nz 0508 800 800 SERVICE Kairanga Marton Taumarunui Woodville -

Planning: Anita Copplestone and Phillip Percy
Report pursuant to s42A Resource Management Act 1991 In the matter of: A Notice of Requirement to construct and operate a new intermodal rail and freight hub on land between Palmerston North and Bunnythorpe And: A hearing by Palmerston North City Council pursuant to s100A Requiring Authority: KiwiRail Holdings Ltd Hearing date: 9 August 2021 S42A Technical Evidence: Planning By: Anita Copplestone and Phillip Percy, Consultant Planners 1 Executive Summary 1.1 Purpose of this report 1. KiwiRail Holdings Limited has lodged a Notice of Requirement for a Regional Freight Hub with Palmerston North City Council. The NOR is for a designation for the construction and operation of an intermodal rail and freight facility. 2. An Independent Hearing Panel (“the Panel”) has been appointed by the Council to make a recommendation on the NOR to the Requiring Authority (KiwiRail). We have prepared this planning report on behalf of the Council to assist the Panel to make a recommendation on the NOR. The report has been prepared in accordance with s 42A of the RMA. 3. The purpose of this report is to identify and crystallise the principal issues, environmental effects and policy that will be considered through the hearing process. In providing this planning advice, we draw on and cross reference the independent advice provided by other experts in the s 42A team. 4. The expert scientific or engineering advice that we have relied on is set out in the following supporting Technical Evidence s 42A reports: a. Technical Report on Traffic and Transport effects – by Harriet Fraser; b. Technical Report on Noise and Vibration effects - by Nigel Lloyd; c. -

State and Trends of River Water Quality in the Manawatū-Whanganui Region
State and Trends of River Water Quality in the Manawatū-Whanganui Region November 2018 Horizons Report 2018/EXT/1619 Prepared for: Abby Matthews November 2018 Science & Innovation Manager Report No. 2018/EXT/1619 ISBN 978-1-98-853744-3 Prepared by: Caroline Fraser and Ton Snelder Client Report 2018-08 For any information regarding this report please contact: Caroline Fraser Phone: 022 049 1779 Email: [email protected] LWP Ltd, PO Box 70 Lyttelton 8092 CONTACT 24 hr Freephone 0508 800 800 [email protected] www.horizons.govt.nz Kairanga Taumarunui Levin Cnr Rongotea and 34 Maata Street 120–122 Hōkio Beach Road Kairanga-Bunnythorpe Roads SERVICE REGIONAL Palmerston North Taihape Palmerston North DEPOTS CENTRES HOUSES 11-15 Victoria Avenue Torere Road Ohotu Marton Whanganui Hammond Street 181 Guyton Street Woodville 116 Vogel Street POSTAL Horizons Regional Council, Private Bag 11025, Manawatū Mail Centre, Palmerston North 4442 F 06 9522 929 ADDRESS State and Trends of River Water Quality in the Manawatū-Whanganui Region For all records up to 30 June 2017 November 2018 Prepared By: Caroline Fraser Ton Snelder For any information regarding this report please contact: Caroline Fraser Phone: 022 049 1779 Email: [email protected] LWP Ltd PO Box 70 Lyttelton 8092 New Zealand LWP Client Report Number: LWP Client Report 2018-08 Report Date: November 2018 LWP Project: 2018-08 Quality Assurance Statement [Click here and type text] Version (2) 11.09.18 Version (1) 31.07.18 Reviewed By Ned Norton [Signature] Page 2 of 126 Table of Contents Executive Summary ................................................................................................... 9 1 Introduction .................................................................................................... -

Wellington Conservation Management Strategy, Volume 1
CMS CONSERVATION MANAGEMENT STRATEGY Wellington 2019, Volume I Cover: McKinnon Hut, 2012. This is a standard six-bunk hut in Ruahine Forest Park. Photographer: Jonathan Astin © Jonathan Astin © January 2019, New Zealand Department of Conservation ISBN Online - 978-1-98-851481-9 ISBN Print - 978-1-98-851482-6 Crown copyright © 2019 This work is licensed under the Creative Commons Attribution 4.0 International licence. In essence, you are free to copy, distribute and adapt the work, as long as you attribute the work to the Crown and abide by the other licence terms. To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/ Please note that no departmental or governmental emblem, logo or Coat of Arms may be used in any way which infringes any provision of the Flags, Emblems, and Names Protection Act 1981. Attribution to the Crown should be in written form and not by reproduction of any such emblem, logo or Coat of Arms. Use the wording ‘Department of Conservation’ in your attribution, not the Department of Conservation logo. This publication is printed on paper pulp sourced from sustainably grown and managed forests, using Elemental Chlorine Free (ECF) bleaching and printed with 100 percent vegetable-based inks. This conservation management strategy is made up of three volumes: Volume I, Volume II Appendices and Volume III Maps. All volumes are online at www.doc.govt.nz/wellingtoncms. Contents Long-term vision for the Wellington region 5 3. National and regional objectives, policies and milestones 26 3.1 Natural values 28 Whakataukī 6 3.2 Historic values 34 3.3 Recreation 38 He kupu whakataki 7 3.4 Engagement 44 3.5 Regional milestones 48 Foreword 7 4. -

Term 4 -21- November -2019
[email protected] www.levin.school.nz Phone 368 6562 Text 02102480530 Levin School Values: Respect, Excellence, Attitude, Co-operation and Honesty. Term 4 Newsletter 21st November 2019 Principal’s Message Kia Ora Koutou, Talofa lava, Malo e lelei, Ni Hao, Chao & Namaste. What fantastic learning opportunities our students have had over the past few weeks. They have experienced…. ‘A visit to Waitarere Beach Surf Life Saving Club where students participated in the NZ Surf Life Saving Beach Education Programme, also a visit to the ‘Fairy Garden’ and exploration of ‘Middle Earth’. The key messages from the Beach Education trip were – ‘Swim between the flags’ and ‘Slip, Slop, Slap and Wrap’. Students have been doing a wide range of follow up activities. A ‘Flying’ visit from the RNZAF where Levin School was visited by an Agusta 109 air force helicopter. Students were blown away by this great experience. Well done to Mrs Taylor and her last years class for the letter writing. A group of 24 Year 5 & 6 students visited the Makahika Outdoor Centre for a day of team building and challenging exercises. The ‘high ropes’ activities were certainly a hit, as was the flying fox. Many thanks to the whanau members and friends of the school who helped in making these events a success. It wouldn’t be possible without your support. “It takes a community to raise a child”. Nga Mihi Paddy Sannazzaro Welcome: Taratoa joined Hinua class last week. We welcome him and his family to Levin School and our Levin School community. Happy Birthday: Kiesha, and Akapei celebrated their birthdays this week. -

01 Front.Pdf (577.2Kb)
Copyright is owned by the Author of the thesis. Pennission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the pennission of the Author. PALEOENVIRONMENTAL ANALYSIS OF QUATERNARY STRATA IN THE LEVIN AREA A thesis presented in partial fulfilment of the requirements for the degree of Master of Science in Quaternary Science at Massey University ALAN HENRY SEWELL 1991 ·-~-. - - ,.-.··,_·.:·.-· Frontispiece: Pencil sketch looking north from the Tararua foothills, south-east of Potts Hill, across the Tokomaru Marine Terrace and Manawatu River flood plain beyond. ABSTRACT Marine transgression during the Last Interglacial resulted in widespread inundation of the southern Manawa tu area. The Otaki Formation constitutes the relatively thick blanket of predominantly marine sand deposited at the height of the transgression and is now exposed in a partially dissected marine terrace abutting the Tararua Range. Sedimentation was controlled by basement block faulting related to a regional strike-slip tectonic regime on the south eastern margin of the South Wanganui Basin. Wave-induced longshore currents from the north-west supplied abundant sediment to the coast. North-east of Levin the Kairanga Trough, occupying a north-east-trending structural depression between uplifted basement blocks, formed the centre of an embayment during the transgression. Tide-dominated depositional processes predominated around the margins of the embayment. In the Forest Lakes area, the absence of seaward barriers resulted in an open wave-dominated coastline. Between Ohau and Shannon mixed wave/tide processes predominated. Stabilisation of sea level resulted in shoreline progradation which was especially marked south of Levin where a dune belt formed, mantling the coastal cliff and later migrating inland. -

Feilding Manawatu Palmerston North City
Mangahuia Wetlands (I2) Rangiwahia Domain (H2) Location: Main South Road, Rangiwahia. Phone: +64 6 328 2840 (See Manawatu Scenic Route) OFFICIAL VISITOR GUIDE OFFICIAL VISITOR GUIDE Website: irongates.co.nz Explore nature’s play land planted with all sorts of interesting trees, shrubs and flowers including water Rangiwahia Environmental Arts Centre (H2) lilies, magnolias and cherry trees. Trout fishing is available in the large and picturesque lake, providing (See Manawatu Scenic Route) you have a licence. There are plenty of birds and wildlife to experience. Free entry. Parking on roadside. Rangiwahia Scenic Reserve (H2) Mangarere Rest Stop and Lookout (G1) (See Manawatu Scenic Route) Location: 6049 State Highway One, Mangaweka. Robotic Dairy Farm Manawatu(F6) A stunning hill top lookout well worth pulling over at. Gaze out towards the white papa cliffs of the Location: Bunnythorpe. Phone: +64 27 632 7451 Rangitikei River. Website: robotfarmnz.wixsite.com/robotfarmnz Take a farm tour and watch the clever cows milk themselves in the amazing robotic milking machines, Mangaweka Campground (G1) experience biological, pasture-based, free-range, sustainable, robotic farming. Bookings essential. Location: 118 Ruahine Road, Mangaweka. Phone: +64 6 382 5744 Website: mangaweka.co.nz An idyllic basic option for a fun Kiwi camp experience with the main office located at Awastone. Great The Coach House Museum (E5) spot for fishing, swimming or just relaxing under the native trees. You can hire a cabin that includes a Location: 121 South Street, Feilding. Phone: +64 6 323 6401 full kitchen, private fire pit and wood-burning BBQ. Website: coachhousemuseum.org Explore the real New Zealand on The Country Road.