Neurological Manifestations of COVID-19 (SARS-Cov-2): a Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Viral Encephalitis and Meningitis
Peachtree Street NW, 15th Floor Atlanta, Georgia 30303-3142 Georgia Department of Public Health www.health.state.ga.us Viral Encephalitis and Viral (Aseptic) Meningitis Frequently Asked Questions What are viral encephalitis and viral meningitis? Viral encephalitis is inflammation (or swelling) of the brain caused by a viral infection. Symptoms of viral encephalitis include headache, fever, stiff neck, seizures, changes in consciousness such as confusion or coma, and sometimes death. Viral (or aseptic) meningitis is also caused by a viral infection resulting in inflammation (or swelling) of the meninges, the protective covering of the brain and spinal cord. The symptoms of viral meningitis are similar to those of viral encephalitis, although loss of or changes in consciousness are not common symptoms of viral meningitis. Viral and bacterial meningitis are not caused by the same organisms, and viral meningitis is usually not as serious as bacterial meningitis. What causes viral encephalitis and viral (aseptic) meningitis? Organisms called viruses cause viral encephalitis and viral meningitis. Many different types of viruses cause these illnesses. Some of these viruses can be passed from person to person, such as when people (especially young children) do not practice good hygiene by washing their hands thoroughly. Other viruses can be passed to people through the bites of infected mosquitoes or ticks. When do most cases of viral encephalitis and viral meningitis occur? Viral encephalitis and viral meningitis occur year‐round. Encephalitis from mosquito bites usually occurs in the late summer and fall, when mosquitoes are most active. Tick‐borne viral encephalitis usually occurs in the spring and early summer, although cases of tick‐borne encephalitis have never been documented in Georgia. -

Death in the Home: Domestic Violence Against Women in Khyber
J Ayub Med Coll Abbottabad 2012;24(1) ORIGINAL ARTICLE DEATH IN THE HOME: DOMESTIC VIOLENCE AGAINST WOMEN IN KHYBER PAKHTUNKHWA Mian Mujahid Shah, Naveed Alam*, Qudsia Hassan**, Sahibdad Khan*, Iftikhar Qayum†, Sher Bahadur†, Zahid Hussain Khalil* Department of Forensic Medicine, Rehman Medical College, *Khyber Medical College, Peshawar, **Ziauddin Medical University Karachi, †Department of Medical Research and Education, Rehman Medical College, Peshawar Background: Domestic or Interpersonal Violence (IPV) remains a major global problem often resulting in morbidity and mortality. The present study was conducted to determine the scope of deaths related to domestic violence in the Khyber Pakhunkhwa province, Pakistan. Methods: Data were collected on all reported female fatalities due to domestic violence for the years 2009–2011 from the records of the department of Forensic Medicine, Khyber Medical College Peshawar for analysis. Results: A total of 305 deaths were reported, showing an increasing trend of 115 deaths for 2009–10 and 190 deaths for 2010–2011. The majority, 182 (59.7%) belonged to the rural areas and 123 (40.3%) to urban areas of the province. Victims were generally of the younger age groups (17% below age 16 and 42.3% between 17–32 years). Homicide was the manner of death in 293 (96.1%) while the most common causative agent was firearm injury (235, 77.1%). Head and neck injuries were most common (52.6%) followed by the chest and abdomen (31.6%) while multiple sites and extremities accounted for 15.8% of injuries. Conclusion: Young and adult females of KPK province of Pakistan are susceptible to homicidal deaths due to domestic violence, perpetrated through firearm injuries to the head and neck regions. -

Download FATA Colleges Choices Form
Pre Merit List Merit No. Declaration of Preferences PMC MDCAT Roll No. MBBS/BDS(FATA/MAD SEATS) for Agency/FR. Public Sector Medical Dental Colleges/Institutions Session 2020-21 VERY IMPORTANT: 1.Write your preferences in the order you would like to be considered for admission against the name of College/Institution. 2.Preference once given shall be final and cannot be changed subsequently. Think carefully before writing. 3.Cutting / erasing / over writing is not allowed. 4.The applicant shall never be considered for a college which he/she has not written down in this list of choices. The University shall not assign a college by itself if the alternate choices are not indicated. 5.Khyber Girls Medical College, Peshawar and Fatima Jinnah Medical College, Lahore are for female students only. Male students cannot opt for this college. 6.Candidate who do not submit choices by the deadline i.e. till 02:00 pm on 17/02/2021, the placement committee shall consider his/her choices recorded previously on the already submitted online application form. Name of the Institution/College Choice No. (In Figure and Words) Signatures of the Applicant ADS=Ayub Dental Section, Abbotabad AMC=Ayub Medical College, Abbotabad BKMC=Bacha Khan Medical College, Mardan BKDS=Bacha Khan Dental Secion, Mardan. BMC=Bannu Medical College, Bannu. GKMC = Gajju Khan Medical College Swabi KGMC = Khyber Girls Medical College (For Girls only) GMC = Gomal Medical College KCD = Khyber College of Dentistry KIMS = KMU Institute of Medical Sciences, Kohat KIDS = KMU Institute of Dental Sciences, Kohat KMC = Khyber Medical College Peshawar 1 of 2 Pre Merit List Merit No. -

Dr. Rafeeq Alam Khan
Rafeeq Alam Khan Curriculum Vitae Meritorious Professor PRESENT STATUS Meritorious Professor Department of Pharmacology Faculty of Pharmacy University of Karachi, Karachi Pakistan Editor in Chief Journal of Basic and Applied Sciences & Journal of Pharmacy and Nutrition Sciences PERSONAL INFORMATION Father's Name Iqbal Alam Khan Date of Birth 09.09.1959 N.I.C. 42101-1807518-9 Marital Status Married Nationality Pakistani E-mail addresses [email protected] , [email protected] Journals website www.lifescienceglobal.com Office Address Department of Pharmacology, Faculty of Pharmacy, University of Karachi, Karachi- 75270 Pakistan Residence B-39, Block-B, Sector 18-A, Gulzar-e-Hijri, Karachi University Employees Co-operative Housing Society, KDA Scheme # 33, Karachi, Pakistan Office Phone +921- 99261300-06 /2361 Cell Number +92-321-8258742 REGISTRATION Registered Pharmacist from Pakistan Pharmacy council Reg. No. 639 dated September 12, 1984. Page 1 of 25 Rafeeq Alam Khan Curriculum Vitae Meritorious Professor EDUCATION Degree Year Specialization/GPA Institute PhD 1996 Pharmacology University of Karachi M. Pharmacy (Gold Medalist) 1986 Pharmacology University of Karachi B. Pharmacy 1983 3.05 University of Karachi REVIEW STATEMENT Twenty nine years of professional, teaching, research and administrative experience had resulted in the creation of a talented team of Pharmacologist not only serving the parent Department, but also enjoying leading positions at various academic institutions throughout Pakistan as well as outside Pakistan. 49 students have been awarded PhD, M. Phil and M. Pharm. degrees under my guidance. I am the editor in chief of two reputable journals. Journal of Basic and Applied Sciences since January 2005, and Journal of Pharmacy and Nutrition Sciences since June 2011, both are now open access journals published by life science global. -

Breast Abcess and Tuberculosis and Its Diagnostic Challenges: a Two-Year Prospective Study in Karachi, Pakistan
Open Access Original Article DOI: 10.7759/cureus.5909 Breast Abcess and Tuberculosis and its Diagnostic Challenges: A Two-Year Prospective Study in Karachi, Pakistan Batool Zehra Asjad 1 , Mir Arsalan Ali 2 , Bushra K. Naeem 3 , Mehmood Khan 1 , Urooj Ahmed Abbasi 4 , Zakia Nehal 5 , Sara Siddiqui 5 1. Surgery, Hamdard University Hospital, Karachi, PAK 2. Surgery, Ziauddin University, Karachi, PAK 3. General Surgery, Jinnah Post Graduate Medical College, Karachi, PAK 4. Surgery, Jinnah Postgraduate Medical Center, Karachi, PAK 5. Surgery, Jinnah Postgraduate Medical Centre, Karachi, PAK Corresponding author: Batool Zehra Asjad, [email protected] Abstract Breast tuberculosis (TB) is rare among extrapulmonary tuberculosis cases, and the diagnosis is usually preceded by a high index of suspicion and findings of granulomatous lesions. We conducted this study to evaluate different clinical presentations of breast TB, including its diagnosis and association with lactation. We also examined the association of breast TB with TB elsewhere in the body and contact history. This prospective, descriptive study was conducted at a tertiary care hospital in Karachi, Pakistan from March 2017 to March 2019. The study population consisted of 100 women of age ranging from 30 to 39 years after 10 patients were lost to follow-up. After providing informed written consent and a histopathology or culture report, participants completed a proforma. We used IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY) to analyze the data, which were kept confidential. Twenty-four patients had diabetes, while 36 patients had no comorbidities. The most consistent symptoms were breast pain (98%), breast lump (89%), fever (83%), and discharge (45%). -

Iqbal Ahmad M
Professor Dr. Iqbal Ahmad M. Sc. (Kar.), Ph. D. (London), D. Sc. Royal Society Fellow Tamgha-e-Imtiaz Department of Pharmaceutical Chemistry Baqai Institute of Pharmaceutical Sciences Baqai Medical University, Karachi ORCID # 0000-0001-8817-3595 POSTAL ADDRESS 51, Deh Tor, Toll Plaza, Super Highway Gadap Road, Karachi-74600. Telephone Off: +92-21-34410293-98 Res: +92-21-34660002 Fax: +92-21-34410439 E-mail: [email protected] [email protected] ACADEMIC QUALIFICATIONS B. Sc. Karachi University 1960 M. Sc. Chemistry Karachi University 1962 Ph. D. Pharmaceutical Chemistry London University 1968 Thesis “A Study of Degradation of Riboflavin and Related Compounds” Postgraduate Diploma Statistics Karachi University 1975 D. Sc. Pharmaceutical Chemistry Karachi University 2014 1 PROFESSIONAL QUALIFICATIONS Registered Pharmacist (Category A) Pharmacy Council of Sind 1976 RESEARCH INTERESTS Drug Analysis, Drug Stability, Preformulation and Formulation Development, Vitamin Interactions in Solution Media, Photochemistry of Medicinal Compounds, Laser Flash Photolysis Studies of Biochemical and Pharmaceutical Systems, Pharmaceutical and Herbal Drug Development. EXPERIENCE POSTDOCTORAL RESEARCH 1. Royal Society Fellow, Department of Biology, Imperial College, London, September 1989–December 1990. Worked with Professor Lord G. Porter, O.M., P.R.S., Nobel Laureate on “Laser Flash Photolysis Studies of Redox Reactions of Photosystem II D1/D2 Cytochrome b 559 Reaction Centres of Higher Plants”. 2. Research Associate, Department of Biochemistry, University of Arizona, Tucson, Arizona 85721, U.S.A., July 1980 - September 1981. Worked with Professor G. Tollin, Regents’ Professor of Biochemistry and Chemistry on “Laser Flash Photolysis Studies of Flavoproteins and Cytochromes”. 3. Postdoctoral Fellow, Research Division, North E. Wales Institute of Higher Education, Connah’s Quay, Clwyd CH5 4BR, U.K., September 1978–June 1980. -

This Podcast on Meningitis Vaccinations for Adolescents
Meningitis Immunization for Adolescents [Announcer] This podcast is presented by the Centers for Disease Control and Prevention. CDC - safer, healthier people. [Susan Laird] Welcome to this podcast on meningitis immunizations for adolescents. I’m Susan Laird, your host. Here to discuss this topic is Dr. Tom Clark, an epidemiologist with CDC’s National Center for Immunization and Respiratory Diseases. Thanks for coming, Dr. Clark. [Dr. Clark] Well, thank you for having me. [Susan Laird] So, tell us about meningococcal disease. [Dr. Clark] Well, meningococcal disease is an infection caused by a bacteria called Neisseria meningitidis. It can be a life-threatening infection. People may be most familiar with this germ as a cause of meningitis, but there are other forms of the disease as well. Meningitis makes up about half of all cases. Other infections can occur, even though they’re less common. For example, bloodstream infection, with or without meningitis and pneumonia can also occur. In meningitis, the infection causes inflammation of the protective fluid and lining around the brain and the spinal cord which are called the meninges. Symptoms include fever, severe headache, neck stiffness, rash, nausea, vomiting, confusion, and sleepiness. Meningitis is serious, so people who develop these symptoms should seek medical attention immediately. [Susan Laird] Is viral meningitis different from bacterial meningitis? [Dr. Clark] It is. Several bacteria can cause meningitis; meningococcal meningitis is one of the most important and most serious. But, meningitis can also be caused by viruses. About 90 percent of viral meningitis is caused by viruses known as enteroviruses. Viral meningitis is more common during the summer and the fall and can be serious, but is rarely fatal in people with normal immune systems. -
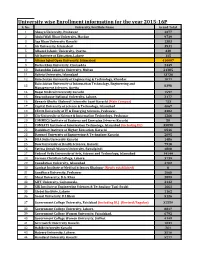
University Wise Enrollment Information for the Year 2015-16P S
University wise Enrollment information for the year 2015-16P S. No. University/Institute Name Grand Total 1 Abasyn University, Peshawar 4377 2 Abdul Wali Khan University, Mardan 9739 3 Aga Khan University Karachi 1383 4 Air University, Islamabad 3531 5 Alhamd Islamic University, Quetta. 338 6 Ali Institute of Education, Lahore 115 8 Allama Iqbal Open University, Islamabad 416607 9 Bacha Khan University, Charsadda 2449 10 Bahauddin Zakariya University, Multan 21385 11 Bahria University, Islamabad 13736 12 Balochistan University of Engineering & Technology, Khuzdar 1071 Balochistan University of Information Technology, Engineering and 13 8398 Management Sciences, Quetta 14 Baqai Medical University Karachi 1597 15 Beaconhouse National University, Lahore. 2177 16 Benazir Bhutto Shaheed University Lyari Karachi (Main Campus) 753 17 Capital University of Science & Technology, Islamabad 4067 18 CECOS University of IT & Emerging Sciences, Peshawar. 3382 19 City University of Science & Information Technology, Peshawar 1266 20 COMMECS Institute of Business and Emerging Sciences Karachi 50 21 COMSATS Institute of Information Technology, Islamabad (including DL) 35890 22 Dadabhoy Institute of Higher Education, Karachi 6546 23 Dawood University of Engineering & Technology Karachi 2095 24 DHA Suffa University Karachi 1486 25 Dow University of Health Sciences, Karachi 7918 26 Fatima Jinnah Women University, Rawalpindi 4808 27 Federal Urdu University of Arts, Science and Technology, Islamabad 14144 28 Forman Christian College, Lahore. 3739 29 Foundation University, Islamabad 4702 30 Gambat Institute of Medical Sciences Khairpur (Newly established) 0 31 Gandhara University, Peshawar 1068 32 Ghazi University, D.G. Khan 2899 33 GIFT University, Gujranwala. 2132 34 GIK Institute of Engineering Sciences & Technology Topi-Swabi 1661 35 Global Institute, Lahore 1162 36 Gomal University, D.I.Khan 5126 37 Government College University, Faislabad (including DL) (Revised/Regular) 32559 38 Government College University, Lahore. -

Khyber Medical University, Peshawar
KHYBER MEDICAL UNIVERSITY, PESHAWAR BACHELOR OF MEDICINE & BACHELOR OF SURGERY (MBBS) THIRD PROFESSIONAL SUPPLEMENTARY EXAMINATION 2011 RECHECKING RESULT DECLARED ON AUGUST 11, 2012 MAX MARKS: 600 NOTIFICATION NO. MBBS (3RD Prof -S11)-2 Roll Reg.No. Name Father's Name Result No. KMU Institute of Medical Sciences, Kohat 9004 2008-KIMS-33 MUHAMMAD YASIR JAN ABDUL QADEEM Re: Path 9008 2008-KIMS-03 MUHAMMAD IJAZ WAHEED WAHEED HUSSAIN 349 Khyber Girls Medical College, Peshawar 9038 2006-KGMC-96 HUDA NAEEM KHAN ARSHAD NAEEM KHAN No Change Khyber Medical College, Peshawar 9065 2003-KMC-193 SYED FAHAD ALI SHAH SYED WALI SHAH BACHA No Change 9070 2007/KMU/KMC/251 ARSALAN KHAN GUL ROSE KHAN Re: Comm Med 9073 2002-KMC-9277 SOHAILA NIAZI SYED SHAH No Change Abbottabad International Medical College, Abbottabad 9114 09-IMC-HU-03 SABA GUL MUHAMMAD ASHAQ KHAN No Change 9115 09-IMC-HU-05 SADAF SULTAN SULTAN AHMAD 342 9118 09-IMC-HU-52 NEHA TANVEER CHAUDHARY TANVEER INAYAT No Change 9120 09-IMC-HU-49 REHMANA HANIF MUHAMMAD HANIF No Change 9125 09-IMC-HU-41 MEHWISH NAZ GHARIB SHAH 335 9128 09-IMC-HU-48 MEHWISH TAJ MOHAMMAD TAJ KHAN TAHIR 351 9129 07-NAD-0033 PALWASHA SHUAIB MUHAMMAD SHUAIB No Change 9130 07-NAD-0013 BEENISH MUKHTAR MOHAMMAD MUKHTAR No Change 9132 07-NAD-0066 TAYYABA SHAUKAT SARDAR M. SHAUKAT Re: Comm Med 9136 06-NAD-6292 HINA SHAMS SHAMS UR REHMAN No Change 9139 09-IMC-HU-31 MUHAMMAD MOHSIN ASLAM ASLAM AHSAN No Change Women Medical College, Abbottabad 9170 2006-WMC-3185 AYESHA ELAHI ELAHI BAKHSH 314 9182 2007-WMC-649 SADIA FAROOQ MUHAMMAD -

Self-Esteem of Patients Receiving Chemotherapy Treatment in a Tertiary Care Hospital Karachi, Pakistan
Self-Esteem of Patients Receiving Chemotherapy Treatment in a Tertiary Care Hospital Karachi, Pakistan SHORT ARTICLES Self-Esteem of Patients Receiving Chemotherapy Treatment in a Tertiary Care Hospital Karachi, Pakistan Daizi Jafar, Fozia Pesnani, Shireen Arif, Alia Nasir Ziauddin University College of Nursing, Karachi, Pakistan. ABSTRACT Background: Cancer is becoming serious and emerging health concern around world. In Pakistan, 8% of all deaths are due to cancer as one of the major cause. Treatment of cancer consists of surgical management, radiation, chemotherapy, hormone therapy, immunotherapy, and combined therapy. Psychological imbalance is observed during treatment and cause altered self-esteem, which requires psychological modification. The study aimed to assess the alteration in self-esteem after receiving chemotherapy. Methods: This research study was cross-sectional study, in which fifty individuals were selected between ages of 18-80 years from oncology unit of tertiary care hospital. A self-administered questionnaire consist of Rosenberg self-esteem scale assessing the self-esteem was administered. Teaching sessions for developmental change towards giving education on increasing self-esteem of patients were conducted for oncology nurses. Results were analyzed using SPSS version 22. Results: Since, 44% male and 56% female were participated. In all surveyed individuals 96% participants identified with average normal self-esteem, 3% participants had low self-esteem, whereas, only 1% participants had high self-esteem. Conclusion: This research study revealed that most of the patient suffering cancer had average self-esteem. Therefore, there is need to work on strategies to promote psychological well-being of patients, aiming to uphold and rehabilitate emotional aspects of cancer patients. -

Viral Meningitis Fact Sheet
Viral ("Aseptic") Meningitis What is meningitis? Meningitis is an illness in which there is inflammation of the tissues that cover the brain and spinal cord. Viral or "aseptic" meningitis, which is the most common type, is caused by an infection with one of several types of viruses. Meningitis can also be caused by infections with several types of bacteria or fungi. In the United States, there are between 25,000 and 50,000 hospitalizations due to viral meningitis each year. What are the symptoms of meningitis? The more common symptoms of meningitis are fever, severe headache, stiff neck, bright lights hurting the eyes, drowsiness or confusion, and nausea and vomiting. In babies, the symptoms are more difficult to identify. They may include fever, fretfulness or irritability, difficulty in awakening the baby, or the baby refuses to eat. The symptoms of meningitis may not be the same for every person. Is viral meningitis a serious disease? Viral ("aseptic") meningitis is serious but rarely fatal in persons with normal immune systems. Usually, the symptoms last from 7 to 10 days and the patient recovers completely. Bacterial meningitis, on the other hand, can be very serious and result in disability or death if not treated promptly. Often, the symptoms of viral meningitis and bacterial meningitis are the same. For this reason, if you think you or your child has meningitis, see your doctor as soon as possible. What causes viral meningitis? Many different viruses can cause meningitis. About 90% of cases of viral meningitis are caused by members of a group of viruses known as enteroviruses, such as coxsackieviruses and echoviruses. -

Complications of Obesity in Polycystic Ovary Syndrome: Insulin Resistance and Inflammation
Complications of Obesity in Polycystic Ovary Syndrome: Insulin Resistance and Inflammation ORIGINAL ARTICLE Complications of Obesity in Polycystic Ovary Syndrome: Insulin Resistance and Inflammation Sadaf Saleem Uppal1, Abdul Khaliq Naveed2, Asifa Majeed3, Mazhar Shafiq4, Kiran Namoos1, Saeeda Baig5 1Department of Biochemistry, Shalamar Medical and Dental College, Lahore, 2Department of Biochemistry, CMH Lahore Medical College, 3Department of Biochemistry and Molecular Biology, Army Medical College, Rawalpindi, 4Department of Radiology, CMH Kohat, 5Department of Biochemistry, Ziauddin University, Karachi, Pakistan. ABSTRACT Background: The polycystic ovarian syndrome (PCOS), a well-known endocrine-metabolic disease, is caused by the interaction of environmental, genetic and metabolic factors. A cross-sectional, comparative study was planned to evaluate high sensitivity C-reactive protein (hs-CRP), insulin and lipid profile in non-obese and obese PCOS patients. Methods: Seventy-two women diagnosed with PCOS as per Rotterdam criteria, were placed in three groups in accordance with the body mass index: Group 1 (normal weight), Group 11 (overweight), and Group 111 (obese). Blood glucose, hs-CRP, serum insulin, lipid profile was estimated and insulin resistance was calculated. Data was analyzed using version 20 of SPSS. Analysis of variance (ANOVA) was used to compare the numeric variables among three groups of PCOS women and p-value < 0.05 was considered statistically significant. Results: Significantly, higher levels of insulin (13.03 ± 0.22), triglyceride (1.74 ± 0.96) and hs-CRP (7.24±4.11) were detected in obese PCOS women. The levels of fasting blood glucose (4.61±0.54) were also raised. Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) (2.69±0.79) showed insulin resistance in obese PCOS women (p=0.004).