Coagulation Monitoring
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

What Does a Diagnosis of Myeloma Mean in 2015
What does a diagnosis of Myeloma mean in 2015 Dr Aristeidis Chaidos Consultant Haematologist Hammersmith Hospital Learning objectives • define myeloma and related disorders • diagnosis & disease monitoring • “many myelomas”: staging & prognostic systems • the evolving landscape in myeloma treatment • principles of management with emphasis to care in the community • future challenges and novel therapies Myeloma – overview • malignancy of the plasma cells (PC): the terminally differentiated, antibody producing B cells • myeloma cells infiltrate the bone marrow • IgG or IgA paraprotein (PP) and/or free light chains in blood and urine • bone destruction • kidney damage • anaemia • despite great advances in the last 15 years myeloma remains an incurable disease Myeloma in figures • 1% of all cancers – 13% of blood cancers • median age at diagnosis: 67 years • only 1% of patients <40 years • 4 – 5 new cases per 100,000 population annually, but different among ethnic groups • 4,800 new myeloma patients in the UK each year • the prevalence of myeloma in the community increases with outcome improvements and aging population Myeloma related conditions smoldering symptomatic remitting plasma cell MGUS refractory myeloma myeloma relapsing leukaemia <10% PC ≥10% PC +/- ≥10% PC or plasmacytoma circulating PC PP <30g/L PP ≥30g/L Any PP in serum and/or urine extramedullary no organ no organ disease damage or damage or organ damage & symptoms symptoms symptoms Death MGUS monoclonal gammopathy of unknown significance • The most common pre-malignant condition: -

Guidelines on the Assessment of Bleeding Risk Prior to Surgery Or Invasive Procedures
guideline Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures British Committee for Standards in Haematology Y. L. Chee, 1 J. C. Crawford, 2 H. G. Watson1 and M. Greaves3 1Department of Haematology, Aberdeen Royal Infirmary, Aberdeen, 2Department of Anaesthetics, Southern General Hospital, Glasgow, and 3Department of Medicine and Therapeutics, School of Medicine, University of Aberdeen, Aberdeen, UK surgery or invasive procedures to predict bleeding risk. The Summary aim is to evaluate the use of indiscriminate testing. Appro- Unselected coagulation testing is widely practiced in the priate testing of patients with relevant clinical features on process of assessing bleeding risk prior to surgery. This may history or examination is not the topic of this guideline. The delay surgery inappropriately and cause unnecessary concern target population includes clinicians responsible for assess- in patients who are found to have ‘abnormal’ tests. In addition ment of patients prior to surgery and other invasive it is associated with a significant cost. This systematic review procedures. was performed to determine whether patient bleeding history and unselected coagulation testing predict abnormal periop- Methods erative bleeding. A literature search of Medline between 1966 and 2005 was performed to identify appropriate studies. The writing group was made up of UK haematologists with Studies that contained enough data to allow the calculation of a special interest in bleeding disorders and an anaesthetist. the predictive value and likelihood ratios of tests for periop- First, the commonly employed coagulation screening tests erative bleeding were included. Nine observational studies were identified and their general and specific limitations (three prospective) were identified. -

Point-Of-Care Testing for Anticoagulation Monitoring In
Point-of-Care Testing for Anticoagulation PATIENT SAFETY Monitoring in Neuroendovascular Procedures H.M. Hussein SUMMARY: POC testing is defined as diagnostic testing at or near the site of patient care. Rapid A.L. Georgiadis measurement of the intensity of anticoagulation and, more recently, platelet inhibition allows dose titration of adjuvant medications such a heparin and antiplatelet agents during neuroendovascular A.I. Qureshi procedures. However, knowledge among practicing physicians regarding the pathophysiologic basis of these measurements and variations in knowledge about the differences among devices is often limited. This review discusses the role of anticoagulation in endovascular procedures and the currently available POC tests for anticoagulation monitoring. ABBREVIATIONS: ACT ϭ activated clotting time; Anti-Xa ϭ quantitative chromogenic heparin assay; aPTT ϭ activated partial thromboplastin time; AT ϭ antithrombin; CI ϭ confidence interval; GP IIb/IIIa ϭ glycoprotein IIb/IIIa; Hemonox-CT ϭ Hemonox clotting time; HIT ϭ heparin-induced thrombocytopenia; HMT ϭ Heparin Management Test; IV ϭ intravenous; LMWH ϭ low-molecular- weight heparin; MI ϭ myocardial infarction; OR ϭ odds ratio; PCI ϭ percutaneous coronary intervention; POC ϭ point of care; UFH ϭ unfractionated heparin hromboembolism ensues from activation of platelets and Brief Overview of Anticoagulant Medications Tthe coagulation cascade and is a major source of compli- Anticoagulants reduce the activity of proteases in the coagula- cations during endovascular procedures. Multiple studies tion cascade. On the basis of their mechanism of action (Fig 1), have demonstrated accelerated platelet activation during cor- anticoagulants can be divided into 4 major groups: vitamin K onary and cerebral angioplasty.1,2 The coagulation cascade antagonists (warfarin), heparins (UFH and LMWH), direct (Fig 1) consists of a sequential conversion of a series of proen- thrombin inhibitors, and direct factor Xa inhibitors. -

Kengrexal, INN-Cangrelor Tetrasodium
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Kengrexal 50 mg powder for concentrate for solution for injection/infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains cangrelor tetrasodium corresponding to 50 mg cangrelor. After reconstitution 1 mL of concentrate contains 10 mg cangrelor. After dilution 1 mL of solution contains 200 micrograms cangrelor. Excipient with known effect Each vial contains 52.2 mg sorbitol. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Powder for concentrate for solution for injection/infusion. White to off-white lyophilised powder. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Kengrexal, co-administered with acetylsalicylic acid (ASA), is indicated for the reduction of thrombotic cardiovascular events in adult patients with coronary artery disease undergoing percutaneous coronary intervention (PCI) who have not received an oral P2Y12 inhibitor prior to the PCI procedure and in whom oral therapy with P2Y12 inhibitors is not feasible or desirable. 4.2 Posology and method of administration Kengrexal should be administered by a physician experienced in either acute coronary care or in coronary intervention procedures and is intended for specialised use in an acute and hospital setting. Posology The recommended dose of Kengrexal for patients undergoing PCI is a 30 micrograms/kg intravenous bolus followed immediately by 4 micrograms/kg/min intravenous infusion. The bolus and infusion should be initiated prior to the procedure and continued for at least two hours or for the duration of the procedure, whichever is longer. At the discretion of the physician, the infusion may be continued for a total duration of four hours, see section 5.1. -

Section 8: Hematology CHAPTER 47: ANEMIA
Section 8: Hematology CHAPTER 47: ANEMIA Q.1. A 56-year-old man presents with symptoms of severe dyspnea on exertion and fatigue. His laboratory values are as follows: Hemoglobin 6.0 g/dL (normal: 12–15 g/dL) Hematocrit 18% (normal: 36%–46%) RBC count 2 million/L (normal: 4–5.2 million/L) Reticulocyte count 3% (normal: 0.5%–1.5%) Which of the following caused this man’s anemia? A. Decreased red cell production B. Increased red cell destruction C. Acute blood loss (hemorrhage) D. There is insufficient information to make a determination Answer: A. This man presents with anemia and an elevated reticulocyte count which seems to suggest a hemolytic process. His reticulocyte count, however, has not been corrected for the degree of anemia he displays. This can be done by calculating his corrected reticulocyte count ([3% × (18%/45%)] = 1.2%), which is less than 2 and thus suggestive of a hypoproliferative process (decreased red cell production). Q.2. A 25-year-old man with pancytopenia undergoes bone marrow aspiration and biopsy, which reveals profound hypocellularity and virtual absence of hematopoietic cells. Cytogenetic analysis of the bone marrow does not reveal any abnormalities. Despite red blood cell and platelet transfusions, his pancytopenia worsens. Histocompatibility testing of his only sister fails to reveal a match. What would be the most appropriate course of therapy? A. Antithymocyte globulin, cyclosporine, and prednisone B. Prednisone alone C. Supportive therapy with chronic blood and platelet transfusions only D. Methotrexate and prednisone E. Bone marrow transplant Answer: A. Although supportive care with transfusions is necessary for treating this patient with aplastic anemia, most cases are not self-limited. -
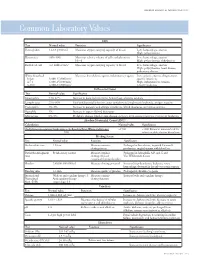
Common Laboratory Values
AmericAn AcAdemy of PediAtric dentistry Reference Manual 2006-2007 Resource Section 251 Common LaboratoryCommon Values Laboratory Values CBC Test Normal value Function Significance Hemoglobin 12-18 g/100 mL Measures oxygen carrying capacity of blood Low: hemorrhage, anemia High: polycythemia Hematocrit 35%-50% Measures relative volume of cells and plasma in Low: hemorrhage, anemia blood High: polycythemia, dehydration Red blood cell 4-6 million/mm3 Measures oxygen-carrying capacity of blood Low: hemorrhage, anemia High: polycythemia, heart disease, pulmonary disease White blood cell Measures host defense against inflammatory agents Low: aplastic anemia, drug toxicity, Infant 8,000-15,000/mm3 specific infections 4-7 y 6,000-15,000/mm3 High: inflammation, trauma, 8-18 y 4,500-13,500/mm3 toxicity, leukemia Differential Count Test Normal value Significance Neutrophils 54%-62% Increase in bacterial infections, hemorrhage, diabetic acidosis Lymphocytes 25%-30% Viral and bacterial infection, acute and chronic lymphocytic leukemia, antigen reaction Eosinophils 1%-3% Increase in parasitic and allergic conditions, blood dyscrasias, pernicious anemia Basophils 1% Increase in types of blood dyscrasias Monocytes 0%-9% Hodgkin’s disease, lipid storage disease, recovery from severe infections, monocytic leukemia Absolute Neutrophil Count (ANC) Calculation Normal value Significance (% Polymorphonuclear Leukocytes + % Bands)×Total White Cell Count >1500 <1000 Patient at increased risk for 100 infection; defer elective dental care Bleeding Screen Test -

Coagulation Testing: High Hematocrit-Anticoagulant Adjustment
PREANALYTIC PULSE Coagulation Testing: High Hematocrit-Anticoagulant Adjustment In 1980, CLSI released H21-A4 guidelines for coagulation testing, which included the recommendation to adjust/correct the amount of citrate in blue-top evacuated blood collection tubes for patients presenting hematocrits greater than 55%. In addition to the CLSI guidelines, the CAP hematology checklist HEM.22830 includes the question, “Are there documented guidelines for the detection and special handling of specimens with elevated hematocrits?” Principle The adjustment is to assure the plasma:anticoagulant ratio (not the blood:anticoagulant ratio) stays consistent. A patient with high hematocrit, greater than 55%, will result in less plasma after centrifugation. The plasma fraction will contain an increased concentration of sodium citrate anticoagulant. The increased concentration may result in falsely prolonged test results for Prothrombin Time (PT) and Activated Partial Thromboplastin Time (aPTT). Formula 1. To calculate the corrected 3.2% sodium citrated whole blood for hematocrit > 55%, adjust the citrate to the proper volume with the following formula: -3 C = (1.85 x 10 )(100-HCT)(Vblood) Where: C = volume of sodium citrate required for that volume of blood HCT = patient’s hematocrit V = volume of blood required in the blood collection tube (example if a 3mL tube is used the blood draw volume is 2.7mL) 1.85 x 10-3 is a constant (considering the citrate volume, blood volume, and citrate concentration). 2. Example: Patient has a Hct of 60% and the patient’s blood will be drawn into a 3mL VACUETTE® sodium citrate (blue-top) blood collection tube. Adjustment of the sodium citrate volume is calculated as: C = (1.85 x 10-3)(100-60)(2.7mL) C = 0.20mL (rounded up from 01.998mL) Remove: 0.30 - 0.20 = 0.10mL of sodium citrate to be removed (0.30 is the difference between the total tube volume of 3.0mL and the blood drawn into the tube of 2.7mL) Method The instructions to prepare an adjusted sodium citrate tube are to be documented in the Laboratory Standard Operating Procedures. -

Prolonged Partial Thromboplastin Time Without Bleeding History; Fletcher Factor Deficiency
Prolonged Partial Thromboplastin Time Without Bleeding History; Fletcher Factor Deficiency Celalettin ÜSTÜN*, Anand JILLELLA*, Linda HENDRIKS*, Mary JONAH**, Ferdane KUTLAR*, Russell BURGESS*, Abdullah KUTLAR* * Section of Hematology Oncology, Department of Medicine, Medical College of Georgia, ** Department of Pathology, Medical College of Georgia, Augusta, USA ABSTRACT A 67-year-old patient was admitted to the hospital to perform an esophagogastrectomy because a lesion at the lower esophagus was strongly suspicious for cancer. Her medical history and her family his- tory were negative for bleeding tendency or thrombosis. Her activated partial thromboplastin time (aPTT) was prolonged (44 s) whereas her prothrombin time (PT) was normal (11 s) presurgery. Mixing of her plasma with normal plasma corrected her prolonged aPTT (27.9 s). Prolonged incubation shortened the patient’s aPTT (36.3 s). Fletcher factor activity was found to be 50%. The patient underwent an esopha- gogastrectomy without bleeding complications under spinal anesthesia. Fletcher factor deficiency, a ra- re disorder, should be considered in patients who have no history of bleeding tendency with a prolonged aPTT. Surgical interventions are safe in these patients. Key Words: aPTT, Surgery, Fletcher factor, Prekallikrein. Turk J Haematol 2002;19(3): 417-419 Received: 20.06.2001 Accepted: 28.07.2001 INTRODUCTION unt[2]. Hematologists are frequently consulted for preoperative coagulation abnormalities. Preoperative evaluation of hemostasis is cru- cial to assess the risk of per-and peri-operative Prolonged aPTT is not an uncommon abnor- bleeding. The most effective screening method is mality encountered during preoperative evaluati- to obtain a thorough history of bleeding[1]. on. It may indicate the presence of antiphospholi- Preoperative screening tests mostly include acti- pid antibodies or a factor deficiency in the intrinsic vated partial thromboplastin time (aPTT), proth- and/or common pathways of blood coagulation. -

Syllabus: Page 23
The University of Texas at El Paso College of Health Sciences Clinical Laboratory Science Program CLSC 3364 Hematology II Course Outline Spring What do you see? What is in your Head? Video or audio recordings will not be permitted. Instructor M. Lorraine Torres, Ed. D, MT (ASCP) College of Health Sciences Room 423 Phone: 747-7282 E-Mail: [email protected] Office Hours TR 3:00 – 4:00 p.m., Friday 2 – 3 p.m. or by appointment Class Schedule Monday and Wednesday 11:00 – 12:30 A.M. HSCI 135 Course Description This course is a sequel to Hematology I. It will include but is not limited to the study of the white blood cells with emphasis on white cell formation and function and the etiology and treatment of white blood cell disorders. This course will also encompass an introduction to hemostasis and laboratory determination of hemostatic disorders. Prerequisite; CLSC 3356 & CLSC 3257. Topical Outline 1. Maturation series and biology of white blood cells 2. Disorders of neutrophils 3. Reactive lymphocytes and Infectious Mononucleosis 4. Acute and chronic leukemias 5. Myelodysplastic syndromes 6. Myeloproliferative disorders 7. Multiple Myeloma and related plasma cell disorders 8. Lymphomas 9. Lipid (lysosomal) storage diseased and histiosytosis 10. Hemostatic mechanisms, platelet biology 11. Coagulation pathways 12. Quantitative and qualitative vascular and platelet disorders (congenital and acquired) 13. Disorders of plasma clotting factors 14. Interaction of the fibrinolytic, coagulation and kinin systems 15. Laboratory methods REQUIRED TEXTBOOKS: same books used for Hematology I Keohane, E.M., Smith, L.J. and Walenga, J.M. 2016. Rodak’s Hematology: Clinical Principles and applications. -

Role of Real-Time / Accurate Diagnostic Testing in Patient Blood Management Programs
ROLE OF REAL-TIME / ACCURATE DIAGNOSTIC TESTING IN PATIENT BLOOD MANAGEMENT PROGRAMS POINT-OF-CARE TESTING “Diagnostic testing is an essential component of Patient Blood Management. The accurate assessment of the true causes of bleeding dysfunction facilitates the employment of evidence based, goal directed therapy to rapidly prevent and treat excess blood loss. “ Sherri Ozawa RN, Clinical Director, Institute of Patient Blood Management, Englewood Hospital Medical Center, Englewood, New Jersey. PATIENT BLOOD MANAGEMENT Interdisciplinary Managing Blood Conservation Anemia Modalities “The timely application of evidence based medical and surgical concepts designed to manage IMPROVED anemia, optimize hemostasis, and PATIENT minimize blood loss in order to OUTCOMES improve patient outcomes.” SABM© 2018 - Society for the Advancement of Blood Management (SABM.org) Optimizing Patient-Centered Coagulation Decision Making Point-of-care testing forms an integral part of PBM, enabling accurate and real-time diagnosis of anemia and bleeding and facilitating precise and targeted hemostatic intervention. 1 Conventional coagulation tests are unable to provide needed information on actual bleeding risk/defects 1, thus delaying appropriate and targeted treatment. Slow turnaround times for laboratory-based conventional coagulation tests are one of the major drivers of empirical treatment regimens, which inevitably result in some patients receiving unnecessary, inappropriate and avoidable transfusions, with associated increased morbidity and mortality, and -

The Role of the Laboratory in Treatment with New Oral Anticoagulants
Journal of Thrombosis and Haemostasis, 11 (Suppl. 1): 122–128 DOI: 10.1111/jth.12227 INVITED REVIEW The role of the laboratory in treatment with new oral anticoagulants T. BAGLIN Department of Haematology, Addenbrooke’s Hospital, Cambridge University Hospitals NHS Trust, Cambridge, UK To cite this article: Baglin T. The role of the laboratory in treatment with new oral anticoagulants. J Thromb Haemost 2013; 11 (Suppl. 1): 122–8. tion of thromboembolism in patients with atrial fibrilla- Summary. Orally active small molecules that selectively tion. For some patients, these drugs offer substantial and specifically inhibit coagulation serine proteases have benefits over oral vitamin K antagonists (VKAs). For the been developed for clinical use. Dabigatran etexilate, majority of patients, these drugs are prescribed at fixed rivaroxaban and apixaban are given at fixed doses and doses without the need for monitoring or dose adjustment. do not require monitoring. In most circumstances, these There are no food interactions and very limited drug inter- drugs have predictable bioavailability, pharmacokinetic actions. The rapid onset of anticoagulation and short half- effects, and pharmacodynamic effects. However, there life make the initiation and interruption of anticoagulant will be clinical circumstances when assessment of the therapy considerably easier than with VKAs. As with all anticoagulant effect of these drugs will be required. The anticoagulants produced so far, there is a correlation effect of these drugs on laboratory tests has been deter- between intensity of anticoagulation and bleeding. Conse- mined in vitro by spiking normal samples with a known quently, the need to consider the balance of benefit and risk concentration of active compound, or ex vivo by using in each individual patient is no less important than with plasma samples from volunteers and patients. -

Approach to Coagulopathy in the Icu Dic and Thrombotic Emergencies
APPROACH TO COAGULOPATHY IN THE ICU DIC AND THROMBOTIC EMERGENCIES NEIL KUMAR, MD UNIVERSITY OF ROCHESTER MEDICAL CENTER Disclosures u I have no financial disclosures u I am NOT A HEMATOLOGIST Outline u Review of hemostasis and coagulopathy u Discuss laboratory markers for coagulopathy u Discuss an approach to a few specific coagulopathies and thrombotic emergencies Outline u Review of hemostasis and coagulopathy u Discuss laboratory markers for coagulopathy u Discuss an approach to a few specific coagulopathies and thrombotic emergencies Coagulation u Coagulation is the process in which blood clots u Fibrinolysis is the process in which clot dissolves u Hemostasis is the stopping of bleeding or hemorrhage. u Ideally, hemostasis is a balance between coagulation and fibrinolysis Coagulation (classic pathways) Michael G. Crooks Simon P. Hart Eur Respir Rev 2015;24:392-399 Coagulation (another view) Gando, S. et al. (2016) Disseminated intravascular coagulation Nat. Rev. Dis. Primers doi:10.1038/nrdp.2016.37 Coagulation (yet another view) u Inflammation and coagulation intersect with platelets in the middle u An example of this is Disseminated Intravascular Coagulation. Gando, S. et al. (2016) Disseminated intravascular coagulation Nat. Rev. Dis. Primers doi:10.1038/nrdp.2016.37 Outline u Review of hemostasis and coagulopathy u Discuss laboratory markers for coagulopathy u Discuss an approach to a few specific coagulopathies and thrombotic emergencies PT / INR u Prothrombin Time u Test of Extrinsic Pathway u Take plasma (blood without cells) and re-add calcium u Calcium was removed with citrate in tube u Add tissue factor u See how long it takes to clot and normalize PT to get INR Coagulation (classic pathways) Michael G.