OXFORD 2020: Meeting the Pandemic Challenge CONTENTS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Old Road Campus
Old Road Campus 4a, 4b, 4c, U5 n o t Oxford City Centre g OLD ROAD n i d 4a, 4b, 4c, U5 a e H K O L L A D R O W AD E M I L 4,4a,4b,4c, U1X,U5 A41 42 4,4a,4b,4c,U5 Rin D 4 g R oad 6 7 1 13 3 11 C H U R C H I L L 900 D 2 R I 700, 900 V E E O x f o 5 A C rd 12 C i ty 10 C e n t re B 8 CAR PARK C 9 h u r c R F h i O l l O S H E o V s N E p L i T t DRI a VE ENTRANCE ROOSEVELT DRIVE l 900, ST2 Index 1 The Triangle Nursery 9 Old Road Campus Estates Annexe 13 Boundary Brook House Interserve Joint Research Office Kennedy Institute 2 - Research Services, Medical Sciences Division Old Road Campus Research Building 10 - Clinical Trials and Research Governance 3 New Richards Building Department of Oncology - Human Tissue Governance CRUK/MRC Oxford Institute for Radiation Oncology - Medical Sciences Division Business Development 4 NDM Research Building Institute of Biomedical Engineering Nuffield Department of Primary Care Health Sciences Target Discovery Institute Jenner Institute Medical Sciences Divisional Safety Officers Centre for Tropical Medicine and Global Health Bodleian Knowledge Centre (Library Services) Medical Sciences Division IT Services 5 Wellcome Centre for Human Genetics (WHG) Ludwig Institute for Cancer Research Structural Genomics Consortium 6 Henry Wellcome Building for Molecular Physiology Nuffield Department of Surgical Sciences Loading Bays and Delivery Offices of the Nuffield Professor of Medicine ENTRANCE VIA BUILDING 5 11 Big Data Institute A Wellcome Trust Centre for Human Genetics 7 Henry Wellcome Building for Particle Imaging -

RTS,S Malaria Vaccine First Malaria Vaccine Will Be Piloted in Areas of Three African Countries Through Routine Immunization Programs
CENTER FOR VACCINE INNOVATION AND ACCESS The RTS,S malaria vaccine First malaria vaccine will be piloted in areas of three African countries through routine immunization programs Summary the vaccine’s role in reducing childhood deaths and severe malaria, and its safety in the context of routine use. Data and Malaria kills more than 400,000 people a year worldwide and information from the MVIP will inform a WHO policy causes illness in tens of millions more, with most deaths recommendation on the broader use of the vaccine. RTS,S has occurring among young children living in sub-Saharan Africa. been approved for use in the pilot evaluation and Phase 4 Although existing interventions have helped to reduce malaria studies by the national regulatory authority in each of the three deaths significantly over the past 15 years, a vaccine could add participating countries. an important complementary tool for malaria control efforts. Financing for the MVIP has been mobilized through an RTS,S/AS01 (RTS,S) is the first malaria vaccine shown to unprecedented collaboration among three global health funding provide partial protection against malaria in young children. It bodies: Gavi, the Vaccine Alliance; the Global Fund to Fight will be the first malaria vaccine provided to young children AIDS, Tuberculosis and Malaria; and Unitaid. Additionally, through national immunization programs in three sub-Saharan WHO, PATH, and GSK are providing in-kind contributions, African countries—Ghana, Kenya, and Malawi. These countries which include GSK’s donation of the vaccine for use in the will introduce the vaccine in selected areas as part of a large- MVIP. -

I Raise the Rates! April Edition
I Raise the Rates! - April Edition I Raise the Rates! April Edition In this edition of I Raise the Rates (IRtR) you will find a variety of new resources from various public health partners, unique education opportunities, and a brief selection of popular media articles related to immunization. Updates from the American College of Physicians (ACP) Opportunity to Participate in ACP Quality Improvement Initiative to Increase Adult Influenza Immunization Rates APPLY NOW - Opportunity to participate in ACP's Quality Improvement Initiative to Increase Adult Influenza Immunization Rates. ACP is recruiting internal medicine and subspecialty practices and residency programs to participate in the I Raise the Rates quality improvement programs to increase influenza and adult immunization rates. ACP’s I Raise the Rates program, which is supported by funding from the CDC, Merck, and GSK, provides QI education and virtual coaching support from ACP Advance expert coaches to support increased adult immunization coverage. The program also offers access to a virtual learning community, tailored educational offerings, and the opportunity to earn more than 54 CME and ABIM MOC credits for program participants. Onboarding is underway so act now! Opportunity is limited, applicants will be considered on a first-come, first-served basis. Please see the attached recruitment flyer for more information about participation benefits and requirements as well as the application link. https://myemail.constantcontact.com/I-Raise-the-Rates----April-Edition---Layout-Template.html?soid=1124874283215&aid=uMyKdB7UvYQ 1/6 I Raise the Rates! - April Edition View the Flyer by Clicking Here ACP COVID-19 Vaccine Forum IV, Practical Clinical Considerations ACP COVID-19 Vaccine Forum IV, Practical Clinical Considerations was the forth in a series of vaccine forums hosted by ACP and Annals of Internal Medicine and was held on March 24, 2021. -

(MGH) COVID-19 Treatment Guidance
Version 8.0 4/28/2021 10:00AM © Copyright 2020 The General Hospital Corporation. All Rights Reserved. Massachusetts General Hospital (MGH) COVID-19 Treatment Guidance This document was prepared (in March, 2020-April, 2021) by and for MGH medical professionals (a.k.a. clinicians, care givers) and is being made available publicly for informational purposes only, in the context of a public health emergency related to COVID-19 (a.k.a. the coronavirus) and in connection with the state of emergency declared by the Governor of the Commonwealth of Massachusetts and the President of the United States. It is neither an attempt to substitute for the practice of medicine nor as a substitute for the provision of any medical professional services. Furthermore, the content is not meant to be complete, exhaustive, or a substitute for medical professional advice, diagnosis, or treatment. The information herein should be adapted to each specific patient based on the treating medical professional’s independent professional judgment and consideration of the patient’s needs, the resources available at the location from where the medical professional services are being provided (e.g., healthcare institution, ambulatory clinic, physician’s office, etc.), and any other unique circumstances. This information should not be used to replace, substitute for, or overrule a qualified medical professional’s judgment. This website may contain third party materials and/or links to third party materials and third party websites for your information and convenience. Partners is not responsible for the availability, accuracy, or content of any of those third party materials or websites nor does it endorse them. -

The 6-In-1 Vaccine Study
Oxford Vaccine Group University of Oxford Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Headington, Oxford OX3 7LE Telephone: 01865 611400 [email protected] www.ovg.ox.ac.uk The 6-in-1 vaccine study Study Information Booklet We are inviting infants aged 8-13 weeks of age to take part in a study comparing two licensed combination vaccines that can be given in the routine UK immunisation schedule. These vaccines protect against 6 different infections in the one injection. Before you decide to take part in this study, it is important for you to understand what the study is about and what participation would involve. Please take time to read the information carefully, and discuss with others if you wish. If you have any questions please contact the study team. Thank you for taking the time to consider this study. Contact the local study team at: Oxford Vaccine Group CCVTM, Churchill Hospital, Oxford, OX3 7LE 01865 611400 [email protected] www.ovg.ox.ac.uk/recruiting-studies Page 1 of 11 The 6-in-1 vaccine study; OVG 2018/05; Participant Information Booklet; REC Ref: 19/SC/0052; IRAS ID: 252324; Version 2.0 dated 23-APR-2019 Oxford Vaccine Group University of Oxford Centre for Clinical Vaccinology and Tropical Medicine, Churchill Hospital, Headington, Oxford OX3 7LE Telephone: 01865 611400 [email protected] www.ovg.ox.ac.uk Summary • This study will help us to better understand the interaction between ‘6- in-1’ vaccines and the Meningococcal B vaccine in the routine UK immunisation schedule • We will be enrolling 240 healthy babies aged 8 – 13 weeks into this study • Babies will receive all their infant immunisations in their own home or in a convenient location until 12 months of age • There will be six study visits including 4 vaccine visits and 2 visits for blood sampling (we will use a topical anaesthetic cream to numb the skin for blood sampling) Why has my child been invited to take part? You have been approached as your child is in the age range for this study and lives in the Thames Valley. -

Assessment Report COVID-19 Vaccine Astrazeneca EMA/94907/2021
29 January 2021 EMA/94907/2021 Committee for Medicinal Products for Human Use (CHMP) Assessment report COVID-19 Vaccine AstraZeneca Common name: COVID-19 Vaccine (ChAdOx1-S [recombinant]) Procedure No. EMEA/H/C/005675/0000 Note Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2021. Reproduction is authorised provided the source is acknowledged. Table of contents 1. Background information on the procedure .............................................. 7 1.1. Submission of the dossier ..................................................................................... 7 1.2. Steps taken for the assessment of the product ........................................................ 9 2. Scientific discussion .............................................................................. 12 2.1. Problem statement ............................................................................................. 12 2.1.1. Disease or condition ........................................................................................ 12 2.1.2. Epidemiology and risk factors ........................................................................... 12 2.1.3. Aetiology and pathogenesis ............................................................................. -

Chadox1 Ncov-19 Vaccine Prevents SARS-Cov-2 Pneumonia in Rhesus Macaques
Article ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques https://doi.org/10.1038/s41586-020-2608-y Neeltje van Doremalen1,4, Teresa Lambe2,4, Alexandra Spencer2, Sandra Belij-Rammerstorfer2, Jyothi N. Purushotham1,2, Julia R. Port1, Victoria A. Avanzato1, Trenton Bushmaker1, Received: 13 May 2020 Amy Flaxman2, Marta Ulaszewska2, Friederike Feldmann3, Elizabeth R. Allen2, Hannah Sharpe2, Accepted: 24 July 2020 Jonathan Schulz1, Myndi Holbrook1, Atsushi Okumura1, Kimberly Meade-White1, Lizzette Pérez-Pérez1, Nick J. Edwards2, Daniel Wright2, Cameron Bissett2, Ciaran Gilbride2, Published online: 30 July 2020 Brandi N. Williamson1, Rebecca Rosenke3, Dan Long3, Alka Ishwarbhai2, Reshma Kailath2, Check for updates Louisa Rose2, Susan Morris2, Claire Powers2, Jamie Lovaglio3, Patrick W. Hanley3, Dana Scott3, Greg Saturday3, Emmie de Wit1, Sarah C. Gilbert2,5 ✉ & Vincent J. Munster1,5 ✉ Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in December 20191,2 and is responsible for the coronavirus disease 2019 (COVID-19) pandemic3. Vaccines are an essential countermeasure and are urgently needed to control the pandemic4. Here we show that the adenovirus-vector-based vaccine ChAdOx1 nCoV-19, which encodes the spike protein of SARS-CoV-2, is immunogenic in mice and elicites a robust humoral and cell-mediated response. This response was predominantly mediated by type-1 T helper cells, as demonstrated by the profling of the IgG subclass and the expression of cytokines. Vaccination with ChAdOx1 nCoV-19 (using either a prime-only or a prime–boost regimen) induced a balanced humoral and cellular immune response of type-1 and type-2 T helper cells in rhesus macaques. -
Astrazeneca-Oxford Vaccine Approved for Use in the U.K
P2JW366000-6-A00100-17FFFF5178F ****** THURSDAY,DECEMBER 31,2020~VOL. CCLXXVI NO.154 WSJ.com HHHH $4.00 DJIA 30409.56 À 73.89 0.2% NASDAQ 12870.00 À 0.2% STOXX 600 400.25 g 0.3% 10-YR. TREAS. À 3/32 , yield 0.926% OIL $48.40 À $0.40 GOLD $1,891.00 À $10.50 EURO $1.2300 YEN 103.21 Deadly Attack at Airport Targets New Yemen Government U.S. IPO What’s News Market Reaches Business&Finance Record nvestorspiled into IPOs Iat a record rate in 2020, with companies raising Total $167.2 billion via 454 of- ferings on U.S. exchanges this year through Dec. 24. Few see signs of letup Few expect the euphoria after companies raise to wear off soon. A1 more than $167 billion Detenteisending in the global fight over tech taxes, despite pandemic with Franceresuming collec- tion of itsdigital-services tax BY MAUREEN FARRELL and the U.S. poised to retali- atewith tariffs.Other coun- Defying expectations,inves- tries areset to join the fray. A1 S tors piled intoinitial public of- China finished 2020 PRES feringsatarecordrateiN with a 10th consecutive TED 2020, and few expect the eu- month of expansion in its CIA phoria to wear off soon. manufacturing sector. A7 SO Companies raised $167.2 AS TheEUand China agreed TENSIONS HIGH: People fled after an explosion Wednesday at the airport in Aden, Yemen, moments after members of the billion through 454 offerings in principle on an invest- country’s newly sworn-in cabinet arrived. At least 22 people were killed, but all the members of the cabinet were safe. -

Transcriptomic Profiling of Dromedary Camels Immunised with a MERS
veterinary sciences Article Transcriptomic Profiling of Dromedary Camels Immunised with a MERS Vaccine Candidate Sharif Hala 1,2,3, Paolo Ribeca 4 , Haya A. Aljami 1,2, Suliman A. Alsagaby 5 , Ibrahim Qasim 6, Sarah C. Gilbert 7 and Naif Khalaf Alharbi 1,2,* 1 Vaccine Development Unit, Department of Infectious Disease Research, King Abdullah International Medical Research Center (KAIMRC), Riyadh 11481, Saudi Arabia; [email protected] (S.H.); [email protected] (H.A.A.) 2 King Saud bin Abdulaziz University for Health Sciences, Riyadh 11481, Saudi Arabia 3 Pathogen Genomics Laboratory, King Abdullah University of Science and Technology (KAUST), Thuwal 23955, Saudi Arabia 4 Biomathematics and Statistics Scotland, The James Hutton Institute, Edinburgh EH9 3FD, UK; [email protected] 5 Department of Medical Laboratory Sciences, College of Applied Medical Sciences, Majmaah University, Al Majmaah 11952, Saudi Arabia; [email protected] 6 Ministry of Environment, Water and Agriculture (MEWA), Riyadh 11481, Saudi Arabia; [email protected] 7 The Jenner Institute, Nuffield Department of Medicine, University of Oxford, Oxford OX1 4BH, UK; [email protected] * Correspondence: [email protected] Abstract: Middle East Respiratory Syndrome coronavirus (MERS-CoV) infects dromedary camels and zoonotically infects humans, causing a respiratory disease with severe pneumonia and death. With no approved antiviral or vaccine interventions for MERS, vaccines are being developed for Citation: Hala, S.; Ribeca, P.; Aljami, camels to prevent virus transmission into humans. We have previously developed a chimpanzee H.A.; Alsagaby, S.A.; Qasim, I.; adenoviral vector-based vaccine for MERS-CoV (ChAdOx1 MERS) and reported its strong humoral Gilbert, S.C.; Alharbi, N.K. -
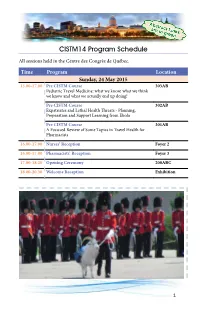
CISTM14 Program Schedule
CISTM14 Program Schedule All sessions held in the Centre des Congrès de Québec. Time Program Location Sunday, 24 May 2015 13.00-17.00 Pre CISTM Course 303AB Pediatric Travel Medicine: what we know, what we think we know and what we actually end up doing! Pre CISTM Course 302AB Expatriates and Lethal Health Threats - Planning, Preparation and Support Learning from Ebola Pre CISTM Course 301AB A Focused Review of Some Topics in Travel Health for Pharmacists 16.00-17.00 Nurses’ Reception Foyer 2 16.00-17.00 Pharmacists’ Reception Foyer 3 17.00-18.20 Opening Ceremony 200ABC 18.00-20.30 Welcome Reception Exhibition 1 Time Program Location Monday, 25 May 2015 MTH1 Meet The History 301AB 8.00-8.45 History of the Quarantine Station at Grosse Ile Marc Desmeules, Canada 8.00-8.45 CDC Yellow Book 302AB Gary Brunette, United States of America COD1 Case of the Day 303AB 8.00-8.45 Pre-Travel Rogelio Lopez-Velez, Spain PL1 Plenary 200ABC 9.00-10.30 Our Shrinking World: Health in the 21st Century Chairs: David R. Shlim, United States of America Leo Visser, The Netherlands PL1.01 Measuring global health: The global is local [Change your mindset! It’s time for a reality check] Louis Loutan, Switzerland • Outline the most important trends and health challenges in the world using gapminder/health metrics • Appraise how these changes (global health transitions, global population growth, urbanization, changes in animal helath and human-animal population interactions, and climate change) will influence global migration and travel medicine now and in the future • Integrate global health concepts into thinking about travel medicine 2 Time Program Location Monday, 25 May 2015, continued Cont. -

Progress in the Development of Potential Therapeutics and Vaccines Against COVID-19 Pandemic
Acta Scientific Pharmaceutical Sciences (ISSN: 2581-5423) Volume 5 Issue 7 July 2021 Review Article Progress in the Development of Potential Therapeutics and Vaccines against COVID-19 Pandemic Abhishek Kumar Yadav, Shubham Kumar and Vikramdeep Monga* Received: May 02, 2021 Department of Pharmaceutical Chemistry, ISF College of Pharmacy, Moga, Punjab, Published: June 09, 2021 India © All rights are reserved by Vikramdeep *Corresponding Author: Vikramdeep Monga, Department of Pharmaceutical Monga., et al. Chemistry, ISF College of Pharmacy, Moga, Punjab, India. Abstract Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes COVID-19 or coronavirus disease 2019 and the same has been declared as a global pandemic by WHO which marked the third introduction of a virulent coronavirus into human society. This a threat to human life worldwide. Considerable efforts have been made for developing effective and safe drugs and vaccines against is a highly pathogenic human coronavirus in which pneumonia of unknown origin was identified in China in December 2019 and is SARS-CoV-2. The current situation and progress in the development of various therapeutic candidates including vaccines in preclini- cal and clinical studies have been described in the manuscript. Until now, many people have been infected with this lethal virus, and a lot of people have died from this COVID-19. This viral disease spreads by coming in contact with an infected person. Understand- ing of SARS-CoV-2 is growing in relation to its epidemiology, virology, and clinical management strategies. Till date, very few drugs or vaccines have been developed or approved for the treatment of this deadly disease of COVID-19 and many candidates are under the clinical development pipeline. -

Dr. Arturo Reyes-Sandoval Associate Professor / Profesor Asociado the Jenner Institute Nuffield Department of Medicine University of Oxford
CURRICULUM VITAE Dr. Arturo Reyes-Sandoval Associate Professor / Profesor Asociado The Jenner Institute Nuffield Department of Medicine University of Oxford Associate Professor at the University of Oxford since 2015. Principal investigator leading a group of scientist composed by 4 postdoctoral scientists, 2 PhD students and a project manager. 56 publications in international journals, H-index 22 and 2133 citations to his research work. 15 grants awarded for a total of £11 million pounds awarded by British Institutions and CONACyT. 8 patents and registrations, 6 as inventor. Educational Qualifications: Degree Award Subject University Year PhD Graduated Doctoral Thesis in Molecular National Polytechnic 2005 with honours Medicine Institute M.Sc. (Hons) Graduated Cytopathology National Polytechnic 1995 with honours Institute University Prize as best Microbiology National Polytechnic 1993 degree student. Institute Finished 1st of 150 students Academic Positions Held: Institution Position Held Start Date End Date University of Oxford Associate Professor 26/03/2015 - The Jenner Institute Wellcome Trust 01/02/2012 01/02/2017 Nuffield Department of Clinical Career development Medicine, University of Oxford Fellow (During this period I received the titles of University Research Lecturer (1 December 2012) and Associate Professor. (26 March 2015). The Jenner Institute Senior Postdoctoral 01/01/2011 31/01/2012 Nuffield Department of Clinical Research Scientist / Medicine, University of Oxford NDM Research Fellow Junior Postdoctoral 24/09/2004 31/12/2010 Research Scientist The Wistar Institute Pre-doctoral trainee 24/09/1999 24/09/2004 Philadelphia Recognition I: Prizes, honours and awards 2016 Nominated by the Mexican Ministry of Foreign Affairs and Mexican Embassy to the UK for the 2016 prize on Science and Technology.