Prevalence and Risk Factors Associated with Lymphatic Filariasis in American Samoa After Mass Drug Administration Shaun P
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Balancing the Tides
JoAnna Poblete Lorem ipsum dolor sit amet, consectetur adipiscing elit. Nam auctor faucibus odio, a scelerisque magna sollicitudin ac. Cras condimentum lacus elementum massa pharetra malesuada. Fusce ac eros varius, viverra urna scelerisque, egestas neque. Fusce tellus arcu, euismod ut rutrum faucibus, elementum quis sem. Fusce auctor sollicitudin lacus, sed sagittis libero tincidunt ac. Praesent vestibulum Balancing tellus a massa ultrices, et mattis augue ultricies. Aliquam at turpis a nisl portti- tor rhoncus id a lacus. Integer a risus eu sapien porta vestibulum. Aenean posu- ere non diam a posuere. Duis laoreet congue tellus, posuere aliquam leo facilisis quis. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Orci varius natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus. Morbi mattis the Tides consequat lacus, ut aliquet dolor accumsan non. Proin suscipit molestie convallis. Vestibulum ante ipsum primis in faucibus orci luctus et ultrices posuere cubilia Curae; Proin quam justo, feugiat non accumsan et, congue sit amet mi. Marine Practices in Vestibulum consectetur mauris et nisl ultrices, eget hendrerit dui vestibulum. Balancing the Mauris gravida sodales nulla eu mattis. Nam tincidunt eu ante vel euismod. Ali- American Sāmoa quam laoreet lacus quis tellus efficitur, vitae porttitor leo pulvinar. Integer dapi- bus gravida quam quis vehicula. Nulla metus nulla, suscipit in porta non, fermen- tum eget urna. Integer quis turpis felis. Sed finibus sem non elementum volutpat. Phasellus pulvinar orci tortor, sed congue elit pharetra eu. Curabitur sodales, dui a luctus semper, metus erat iaculis risus, ut facilisis leo mi vitae lorem. Pellentesque elementum dui ex, in pulvinar nibh maximus ut. -

Download Press Release
FEMA registrations for Aua, Alega, Avaio, Lauli’i, Afono, and Auto Release Date: April 10, 2018 PAGO PAGO, American Samoa – The American Samoa Government (ASG) and FEMA schedule more residents to register for assistance. Only heads of households need to register. Residents who were affected by Tropical Storm Gita may use the following schedule to register for assistance at the Disaster Recovery Center (DRC). Thursday, April 12, Aua all who preregistered Thursday, April 12, Alega and Avaio all who preregistered Friday, April 13, Lauli’i all who preregistered Friday, April 13, Afono all who preregistered Friday, April 13, Auto all who preregistered Saturday, April 14, Make-up day for Mapusaga, Tafeta, Taputimu, Leone, Fagasa, Vatia, Aumi, Aua, Alega, Avaio, Lauli’i, Afono, and Auto. Applicants should have the following information at hand: Social Security number. Address of the damaged primary residence. Description of the damage. Information about insurance coverage if applicable. A current contact telephone number. A PO Box where they can receive mail. Bank account and routing numbers for direct deposit of funds. The Territory and FEMA will continue to schedule dates and times for villages to register for federal assistance at the DRC. Disaster Survivor Assistance (DSA) teams will be available through Monday, April 16 to help register heads of household with access and functional needs. Call the Crisis Hotline at (684) 633-901 to request a home visit. After April 16, DSA teams will not be available. Anyone who already has a slip with DSA written on it is already registered. Page 1 of 2 Page printed at fema.gov/press-release/20210318/fema-registrations-aua-alega-avaio-laulii-afono-and-auto 09/29/2021 The DRC is located at the Department of Youth and Women Affairs, Pago Pago Sports Village, Pago Pago, AS 96799. -

Am. Samoa Hazard Mitigation Plan, 2008
American Samoa Revision and Update of the Territory Hazard Mitigation Plan April 28, 2008 2007 Project Team Territorial Emergency Management Coordinating Office Office of the Governor P.O. Box 997755 Pago Pago, American Samoa 96799 Phone: 011 (684) 699-6482 Fax: 011 (684) 699-6414 Email: [email protected] Website: http://www.asg-gov.com/agencies/temco.asg.htm American Samoa Disaster Recovery Office American Samoa Government Nu'uuli #26-500 P.O. Box 50018 Pago Pago, American Samoa 96799 Phone: 011 (684) 699-1330 Fax: 011 (684) 699-5005 Email: [email protected] Website: http://www.asg-gov.com Jamie Caplan Consulting LLC 351 Pleasant Street, #208 Northampton, MA 01060 USA Phone: 1(413) 586-0867 Fax: 1(413) 303-0412 Email: [email protected] Website: http://www.jamiecaplan.com 2003 Project Team Territorial Emergency Management Coordinating Office Office of the Governor P.O. Box 997755 Pago Pago, American Samoa 96799 Phone: 011 (684) 699-6482 Fax: 011 (684) 699-6414 Email: [email protected] Website: http://www.asg- gov.com/agencies/temco.asg.htm East West Center 1601 East-West Road Honolulu, HI 96848-1601 USA Phone: 1 (808) 944-7111 Fax: 1 (808) 944-7376 Email: [email protected] Website: http://www.EastWestCenter.org Pacific Disaster Center 1305 N. Holopono St. #2 Kihei, HI, 96753-6900 USA Phone: 1 (808) 891-0525 Fax: 1 (808) 891-0526 Email: [email protected] Website: http://www.pdc.org Social Science Research Institute University of Hawaii at Manoa 2424 Maile Way Saunders Hall, Suite 704 Honolulu, HI 96822 USA Phone: 1 (808) 956-8930 Fax: 1 (808) 956-2884 Email: [email protected] Website: http://www.socialsciences.hawaii.edu/ssri/ The 2007 American Samoa Hazard Mitigation Plan was developed by a consulting team from Jamie Caplan Consulting LLC, and the Lieutenant Governor, the Territorial Emergency Management Coordinating Office, the staff of the American Samoa Disaster Recovery Office, the Territorial Hazard Mitigation Council and Directors and technical staff of the American Samoa Government. -

Ethnographic Assessment and Overview National Park of American Samoa
PACIFIC COOPERATIVE STUDIES UNIT UNIVERSITY OF HAWAI`I AT MĀNOA Dr. David C. Duffy, Unit Leader Department of Botany 3190 Maile Way, St. John #408 Honolulu, Hawai’i 96822 Technical Report 152 ETHNOGRAPHIC ASSESSMENT AND OVERVIEW NATIONAL PARK OF AMERICAN SAMOA November 2006 Jocelyn Linnekin1, Terry Hunt, Leslie Lang and Timothy McCormick 1 Email: [email protected]. Department of Anthropology, University of Connecticut Beach Hall Room 445, U-2176 354 Mansfield Road Storrs, Connecticut 06269-2176 Ethnographic Assessment and Overview The National Park of American Samoa Table of Contents List of Tables and Figures iii List of Slides v Preface: Study Issues vi Maps vii Key to Maps x I. The Environmental Context 1 Climate and Vegetation 1 The National Park Environments 4 II. Archaeology and Samoan Prehistory 8 Early Settlement 8 Later Inland Settlement 9 Late Prehistoric Period 9 European Contact and the Historical Period 10 Archaeology in the National Park Units 10 III. Research Methodology 15 Documentary Phase 15 Field Research 15 Limitations of the Research 17 IV. Ethnohistory 22 Myths and Legends Relevant to the Park 22 The European Contact Period 25 Western Ethnohistorical and Ethnographic Reports 31 V. Agriculture and Domestically Useful Plants 46 Tutuila Unit 46 Ta'u Unit 49 Ofu Unit 51 Summary 52 VI. Marine Resources 53 Tutuila Unit 53 Ta'u Unit 57 Ofu Unit 58 Summary 61 i VII. Medicinal Plants 63 Ofu Unit 63 Ta'u Unit 66 Tutuila Unit 66 Summary 67 VIII. Analysis of Freelist Data 75 Crops and Cultivated Plants 76 Medicinal Plants 81 Fish and Marine Species 84 Animals and Birds 86 Summary of the Freelist Results 88 IX. -

CHAPTER II Planning Area Profile for Hazard Mitigation Analysis
CHAPTER II Planning Area Profile for Hazard Mitigation Analysis 24 Territory of American Samoa Multi-Hazard Mitigation Plan A U.S. Territory since 1900, American Samoa is located in the central South Pacific Ocean, 2,300 miles south-southwest of Hawaii and 1,600 miles east-northeast of New Zealand. American Samoa has a total land area of approximately 76 square miles and consists of a group of five volcanic islands and two atolls (Rose Atoll and Swains Island). The five volcanic islands, Tutuila, Aunu’u, Ofu, Olosega, and Ta’u, are the major inhabited islands. Tutuila is the largest island and the center of government. Ofu, Olosega, and Ta’u, collectively are referred to as the Manu’a Islands. Figure 1 Base Map of American Samoa depicts all of the islands of American Samoa. Figure 1. Base Map of American Samoa. The five volcanic islands, Tutuila, Aunu’u, Ofu, Olosega, and Ta’u, are the inhabited islands. At 53 square miles, Tutuila is the largest and oldest of the islands, and is the center of government and business. It is a long, narrow island lying SW-NE, is just over 20 miles in length, and ranges from 1 to 2 miles wide in the eastern half, and from 2 to 5 miles wide in the western half. Home to 95 percent of the territory’s 55,000 residents, Tutuila is the historic capitol (Pago Pago), the seat of American Samoa’s legislature and judiciary (Fagatogo), as well as the office of the Governor. Tutuila is often divided into 3 regions: the eastern district, the western district and Manu’a district. -

Revised Constitution of American Samoa
AMERICAN SAMOA CONSTITUTION REVISED CONSTITUTION OF AMERICAN SAMOA Article I Bill of Rights Section 1. Freedom of religion, speech, press, rights of assembly and petition. 2. No deprivation of life, liberty or property without due process. 3. Policy protective legislation. 4. Dignity of the individual. 5. Protection against unreasonable searchesand seizures. 6. Rights of an accused. 7. Habeas corpus. 8. Quartering of militia. 9. Imprisonment for debt. 10. Slavery prohibited. 11. Treason. 12. Subversives ineligible to hold public office. 13. Retroactive laws and bills of attainder. 14. Health, safety, morals and general welfare. 15. Education. 16. Unspecified rights and privileges and immunities. Article II The Legislature 1. Legislature. 2. Membership. 3. Qualifications of members. 4. Manner of election. 5. Elections. 6. Term of office. 7. Qualifications of electors. 8. Legislative sessions. 9. Enactment of law; vetoes. 10. Passage of bills. 11. Powers of each house. 12. Freedom from arrest. 13. Vacancies. 14. Public sessions. 15. Reading-Passage of bills. 16. Title 17. Amendments and revisions by reference. 18. Appointment to new offices. 19. Effective date of laws. 20. Legislative counsel 21. Quorum. 22. Qualifications and officers. 23. Adjourning Legislature. 24. Special or exclusive privileges not to be granted; local or special laws. 25. Compensation of the Legislature. Article III Judicial Branch 1. Judicial power. 2. Independence of the courts. 3. Appointments. Article IV Executive Branch 1. Appointments. 2. Governor. 3. Secretary. 4. Secretary of Samoan Affairs. 5. Militia and posse comitatus. 6. Executive regulations. 7. Supervision and control by Governor. 8. Annual report. 9. Pardoning power. 10. Recommendation of laws. -

United States Department of the Interior Geological Survey the October 28, 1979, Landsliding on Tutuila, American Samoa by Jane
United States Department of the Interior Geological Survey The October 28, 1979, Landsliding On Tutuila, American Samoa by Jane M. Buchanan-Banks Open-File Report 81-81 This report is preliminary and has not been edited or reviewed for conformity with U. S. Geological Survey editorial standards and stratigraphic nomenclature The October 28, 1979, Landsliding on Tutuila, American Samoa Abstract In an apparently short time interval in the early morning of October 28, 1979, more than 70 landslides occurred on Tutuila Island, American Samoa. They were confined to the western end of the island from Asili on the south shore to Massacre Bay on the north. One of the landslides in Seetaga caused four fatalities; the others caused property damage and blocked the main transportation route on the west end of the island. Although there were rumors of an earthquake immediately preceding the landsliding, an examination of the seismograph from Apia Observatory, Western Samoa, for the morning of October 28 shows no record of an earth quake. Heavy rains during the preceding few days coupled with the general ly steep slopes and deeply weathered geologic materials appear to have caused the slope failures. There is abundant evidence both visually and on aerial photographs taken in 1941 and 1960 that landslides are frequent occurrences in the effected area. It is recommended that an analysis be made of the regional slope stability relative to current and proposed land use practices. Such an analysis would include: 1) air photos taken at a scale of 1:24,000 for comparison with those taken previously to document areas of landsliding; 2) a study of the vegetative cover; 3) a slope map; and 4) a detailed geological and structural map. -

ALOFAU Village 14.263453S 170.619074W 170.591294W
14.263453S CENSUS 2000 BLOCK MAP: ALOFAU Village 14.263453S 170.619074W 170.591294W LEGEND SYMBOL NAME STYLE INTERNATIONAL AIR (FEDERAL) Trust Land / Home Land OTSA / TDSA / ANVSA AMERICAN INDIAN TRIBAL SUBDIVISION AIR (State) SDAISA Masefau ANRC 54500 Masausi 53700 MINOR CIVIL DIV. / CCD 1 Consolidated City Place within Subject Entity Incorporated Place / CDP 1 Place outside of Subject Entity Incorporated Place / CDP 1 Census Tract 2 ABBREVIATION REFERENCE: AIR = American Indian Reservation; Trust Land = Off−Reservation Trust Land; Home Land = Hawaiian Home Land; OTSA = Oklahoma Tribal Statistical Area; TDSA = Tribal Designated Statistical Area; ANVSA = Alaska Native Village Statistical Area; SDAISA = State Designated American Indian Statistical Area; ANRC = Alaska Native Regional Corporation; CDP = Census Designated Place; Minor Civil Div. (MCD) = Minor Civil Division; CCD = Census County Division FEATURES Sa'ilele 66500 Aoa 12100 VAIFANUA CO 86500 Military Inset Out Area Where international, state, and/or county boundaries coincide, the map shows the boundary symbol for only the highest−ranking of these boundaries. 1 A ' * ' following a place name indicates that the place is coextensive with a MCD, A ' ° ' indicates that the place is also a false MCD; the false MCD name is not shown. 2 A ' * ' following a block number indicates that the block number is repeated elsewhere in the block. SA'OLE CO 67300 Pagai 62230 SUA CO 72100 3007 Faga'itua 23300 3000 3006 Tutuila Island Fagaitua Bay 3005 Alofau SA'OLE CO 07300 67300 3002 3001 Amouli 10500 9502 3004 3003 Key to Adjacent Sheets 1 SHEET 1 OF 1 PARENT SHEET Amaua 09700 TOTAL SHEETS: 1 (Index 0; Parent 1; Inset 0) 14.290360S 14.290360S 170.619074W 170.591294W N ENTITY TYPE: Incorporated Place .2 .1 0 .2 mi W E NAME: Alofau village (07300) ST: American Samoa (60) S .3 .2 .1 0 .3 km CO: Eastern Dis (010) All legal boundaries and names are as of January 1, 2000. -

National List of Beaches 2004 (PDF)
National List of Beaches March 2004 U.S. Environmental Protection Agency Office of Water 1200 Pennsylvania Avenue, NW Washington DC 20460 EPA-823-R-04-004 i Contents Introduction ...................................................................................................................... 1 States Alabama ............................................................................................................... 3 Alaska................................................................................................................... 6 California .............................................................................................................. 9 Connecticut .......................................................................................................... 17 Delaware .............................................................................................................. 21 Florida .................................................................................................................. 22 Georgia................................................................................................................. 36 Hawaii................................................................................................................... 38 Illinois ................................................................................................................... 45 Indiana.................................................................................................................. 47 Louisiana -
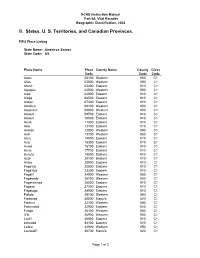
American Samoa State Code: AS
NCHS Instruction Manual Part 8A, Vital Records Geographic Classification, 2004 II. States, U. S. Territories, and Canadian Provinces. FIPS Place Listing State Name: American Samoa State Code: AS Place Name Place County Name County Class Code Code Code Aasu 00100 Western 050 C1 Afao 02500 Western 050 C1 Afono 03300 Eastern 010 C1 Agugulu 04500 Western 050 C1 Alao 04900 Eastern 010 C1 Alega 06500 Eastern 010 C1 Alofau 07300 Eastern 010 C1 Amaluia 08100 Western 050 C1 Amanave 08900 Western 050 C1 Amaua 09700 Eastern 010 C1 Amouli 10500 Eastern 010 C1 Anua 11300 Eastern 010 C1 Aoa 12100 Eastern 010 C1 Aoloau 12800 Western 050 C1 Asili 13700 Western 050 C1 Atu'u 14500 Eastern 010 C1 Aua 15300 Eastern 010 C1 Auasi 16100 Eastern 010 C1 Aumi 17700 Eastern 010 C1 Aunu'u 18500 Eastern 010 C1 Auto 20100 Eastern 010 C1 Avaio 20900 Eastern 010 C1 Faga'alu 22500 Eastern 010 C1 Faga'itua 23300 Eastern 010 C1 Fagali'i 24900 Western 050 C1 Fagamalo 25700 Western 050 C1 Faganeanea 26500 Eastern 010 C1 Fagasa 27300 Eastern 010 C1 Fagatogo 28900 Eastern 010 C1 Failolo 29700 Western 050 C1 Faleasao 30500 Manu'a 020 C1 Faleniu 32100 Western 050 C1 Fatumafuti 32900 Eastern 010 C1 Futiga 36100 Western 050 C1 Ili'ili 36900 Western 050 C1 Lauli'i 40800 Eastern 010 C1 Leloaloa 44100 Eastern 010 C1 Leone 44900 Western 050 C1 Leusoali'i 45700 Manu'a 020 C1 Page 1 of 2 NCHS Instruction Manual Part 8A, Vital Records Geographic Classification, 2004 II. States, U. -

Download Map (PDF | 910.93
This map illustrates satellite-detected areas of likely building and vegetation damages caused by the magnitude 8.0 earthquake and resulting tsunami on 29 September 2009. Disaster coverage by the Earthquake 20 October 2009 A total of 11 damage sites were identified based on post-disaster satellite imagery International Charter 'Space and & Tsunami collected on 2-3 October 2009. Each site has a corresponding high resolution focus Major Disasters'. For more information on the Charter, which map within the PDF document. Damage identification was significantly restricted by is about assisting the disaster Version 1.0 heavy cloud and shadow coverage, as well as by spatial resolution of the imagery. It is relief organizations with multi- probable therefore that damages have been underestimated. This assessment is a satellite data and information, visit Glide No: preliminary analysis & has not yet been validated in the field. Please send ground www.disasterscharter.org !% !6 Damage Analysis with Formosat-2 Data Acquired on 2-3 October 2009 feedback to UNITAR / UNOSAT. !! TS-2009-000209-ASM 170°50'0"W 170°47'30"W 170°45'0"W 170°42'30"W 170°40'0"W 170°37'30"W 170°35'0"W NOTE: DAMAGE IDENTIFICATION WAS SIGNIFICANTLY RESTRICTED BY HEAVY CLOUDS AND SHADOW - SOUTHERN & EASTERN COASTS NOT ASSESSED Vatia Amalau Tafeu Bay Bay Cove Vatia 14°15'0"S Afona Masefau Tula 14°15'0"S Bay Bay Onenoa Pago Pago Masefau Port Sa'ilele Facility Afono Masausi Aoa Alao Leloaloa Fagaitua Pagai Anua Atuu Utulei Amaua Faga'itua D Pago Beach Aua Auasi Utumea T Bay TD Pago -

American Samoa's Marine Protected Area System
JOURNAL OF INTERNATIONAL WILDLIFE LAW & POLICY 2016, VOL. 19, NO. 4, XXX-XXX _____________________________________________________________________________ American Samoa’s Marine Protected Area System: Institutions, Governance, and Scale Jeremy M. Raynal,a Arielle S. Levine,b and Mia T. Comeros-Raynalc 1. Introduction Marine protected areas (MPAs) are a primary marine conservation strategy in the U.S. territory of American Samoa, which has a goal to protect 20% of its coral reef area under “no-take” MPAs. The territory implements MPAs by using diverse governance approaches involving a range of institutions operating at different scales and including federal, territorial, and local village entities. This innovative approach to management takes advantage of the territory’s traditional marine tenure system while drawing upon resources available from the U.S. federal government. Since 2000, total MPA coverage in American Samoa has expanded to encompass approximately 25% of coral reef area in the territory, with nearly 7% of reefs in no-take reserves. This represents a level of resource protection and inter- institutional collaboration that is unusual in the Pacific, and indeed worldwide.1 CONTACT Jeremy M. Raynal [email protected], Coral Reef Advisory Group, P.O. Box 3730 Pago Pago, American Samoa, AS 96799 a Coral Reef Advisory Group, American Samoa. b Department of Geography, San Diego State University, 5500 Campanile Drive, San Diego CA 92182-4493, USA, and NOAA Coral Reef Conservation Program. 2 However, the territory still falls far short of its stated goal. This paper is the first comprehensive description and governance analysis of the American Samoa MPA system, exploring the unique institutional arrangements that have been established, with traditional Samoan governance systems operating c IUCN Global Species Programme/Biological Sciences, Old Dominion University, Norfolk, VA 23529, USA, and American Samoa Environmental Protection Agency, Pago Pago, AS.