Mini-E: a Tractable System for Chromosome and BAC Engineering
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Highly Efficient Recombineering-Based Method for Generating Conditional Knockout Mutations Pentao Liu, Nancy A
Methods A Highly Efficient Recombineering-Based Method for Generating Conditional Knockout Mutations Pentao Liu, Nancy A. Jenkins, and Neal G. Copeland1 Mouse Cancer Genetics Program, Center for Cancer Research, National Cancer Institute, Frederick, Maryland 21702, USA Phage-based Escherichia coli homologous recombination systems have recently been developed that now make it possible to subclone or modify DNA cloned into plasmids, BACs, or PACs without the need for restriction enzymes or DNA ligases. This new form of chromosome engineering, termed recombineering, has many different uses for functional genomic studies. Here we describe a new recombineering-based method for generating conditional mouse knockout (cko) mutations. This method uses homologous recombination mediated by the phage Red proteins, to subclone DNA from BACs into high-copy plasmids by gaprepair,and together with Cre or Flpe recombinases, to introduce loxPorFRT sites into the subcloned DNA. Unlike other methods that use short 45–55-bpregions of homology for recombineering, our metho d uses much longer regions of homology. We also make use of several new E. coli strains, in which the proteins required for recombination are expressed from a defective temperature-sensitive prophage, and the Cre or Flpe recombinases from an arabinose-inducible promoter. We also describe two new Neo selection cassettes that work well in both E. coli and mouse ES cells. Our method is fast, efficient, and reliable and makes it possible to generate cko-targeting vectors in less than 2 wk. This method should also facilitate the generation of knock-in mutations and transgene constructs, as well as expedite the analysis of regulatory elements and functional domains in or near genes. -
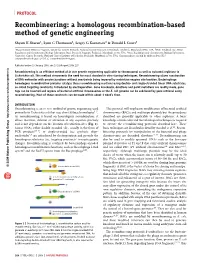
A Homologous Recombination-Based Method of Genetic Engineering
PROTOCOL Recombineering: a homologous recombination-based method of genetic engineering Shyam K Sharan1, Lynn C Thomason2, Sergey G Kuznetsov1 & Donald L Court3 1Mouse Cancer Genetics Program, Center for Cancer Research, National Cancer Institute at Frederick, Frederick, Maryland 21702, USA. 2SAIC-Frederick Inc., Gene Regulation and Chromosome Biology Laboratory, Basic Research Program, Frederick, Maryland, 21702, USA. 3Gene Regulation and Chromosome Biology Laboratory, Center for Cancer Research, National Cancer Institute at Frederick, Frederick, Maryland 21702, USA. Correspondence should be addressed to S.K.S. ([email protected]) or D.L.C. ([email protected]). Published online 29 January 2009; doi:10.1038/nprot.2008.227 Recombineering is an efficient method of in vivo genetic engineering applicable to chromosomal as well as episomal replicons in Escherichia coli. This method circumvents the need for most standard in vitro cloning techniques. Recombineering allows construction of DNA molecules with precise junctions without constraints being imposed by restriction enzyme site location. Bacteriophage homologous recombination proteins catalyze these recombineering reactions using double- and single-stranded linear DNA substrates, natureprotocols so-called targeting constructs, introduced by electroporation. Gene knockouts, deletions and point mutations are readily made, gene / m tags can be inserted and regions of bacterial artificial chromosomes or the E. coli genome can be subcloned by gene retrieval using o c . recombineering. Most of these constructs can be made within about 1 week’s time. e r u t a n . INTRODUCTION w w Recombineering is an in vivo method of genetic engineering used This protocol will emphasize modification of bacterial artificial w / / 1–5 : primarily in Escherichia coli that uses short 50 base homologies . -

Bacterial Artificial Chromosome Mutagenesis Using Recombineering
Hindawi Publishing Corporation Journal of Biomedicine and Biotechnology Volume 2011, Article ID 971296, 10 pages doi:10.1155/2011/971296 Review Article Bacterial Artificial Chromosome Mutagenesis Using Recombineering Kumaran Narayanan1, 2 and Qingwen Chen2 1 Department of Genetics and Genomic Sciences, Mount Sinai School of Medicine, New York, NY 10029, USA 2 School of Science, Monash University, Sunway Campus, Room 2-5-29, Bandar Sunway, 46150, Malaysia Correspondence should be addressed to Kumaran Narayanan, [email protected] Received 2 August 2010; Accepted 21 October 2010 Academic Editor: Masamitsu Yamaguchi Copyright © 2011 K. Narayanan and Q. Chen. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Gene expression from bacterial artificial chromosome (BAC) clones has been demonstrated to facilitate physiologically relevant levels compared to viral and nonviral cDNA vectors. BACs are large enough to transfer intact genes in their native chromosomal setting together with flanking regulatory elements to provide all the signals for correct spatiotemporal gene expression. Until recently, the use of BACs for functional studies has been limited because their large size has inherently presented a major obstacle for introducing modifications using conventional genetic engineering strategies. The development of in vivo homologous recombination strategies based on recombineering in E. coli has helped resolve this problem by enabling facile engineering of high molecular weight BAC DNA without dependence on suitably placed restriction enzymes or cloning steps. These techniques have considerably expanded the possibilities for studying functional genetics using BACs in vitro and in vivo. -

Genetic Engineering Using Homologous Recombination1
17 Oct 2002 13:49 AR AR174-GE36-14.tex AR174-GE36-14.SGM LaTeX2e(2002/01/18) P1: IBC 10.1146/annurev.genet.36.061102.093104 Annu. Rev. Genet. 2002. 36:361–88 doi: 10.1146/annurev.genet.36.061102.093104 GENETIC ENGINEERING USING HOMOLOGOUS RECOMBINATION1 Donald L. Court, James A. Sawitzke, and Lynn C. Thomason Gene Regulation and Chromosome Biology Laboratory, Center for Cancer Research, National Cancer Institute at Frederick, Frederick, Maryland 21702; e-mail: [email protected], [email protected], [email protected] Key Words DNA replication forks, strand annealing, in vivo cloning, oligo recombination, recombineering ■ Abstract In the past few years, in vivo technologies have emerged that, due to their efficiency and simplicity, may one day replace standard genetic engineering tech- niques. Constructs can be made on plasmids or directly on the Escherichia coli chromo- some from PCR products or synthetic oligonucleotides by homologous recombination. This is possible because bacteriophage-encoded recombination functions efficiently re- combine sequences with homologies as short as 35 to 50 base pairs. This technology, termed recombineering, is providing new ways to modify genes and segments of the chromosome. This review describes not only recombineering and its applications, but also summarizes homologous recombination in E. coli and early uses of homologous recombination to modify the bacterial chromosome. Finally, based on the premise that phage-mediated recombination functions act at replication forks, specific molecular models are -

Improved Bacterial Recombineering by Parallelized Protein Discovery
Improved bacterial recombineering by parallelized protein discovery Timothy M. Wanniera,1, Akos Nyergesa,b, Helene M. Kuchwaraa, Márton Czikkelyb, Dávid Baloghb, Gabriel T. Filsingera, Nathaniel C. Bordersa, Christopher J. Gregga, Marc J. Lajoiea, Xavier Riosa, Csaba Pálb, and George M. Churcha,1 aDepartment of Genetics, Harvard Medical School, Boston, MA 02115; and bSynthetic and Systems Biology Unit, Institute of Biochemistry, Biological Research Centre, Szeged HU-6726, Hungary Edited by Susan Gottesman, National Institutes of Health, Bethesda, MD, and approved April 17, 2020 (received for review January 30, 2020) Exploiting bacteriophage-derived homologous recombination pro- recombineering, is the molecular mechanism that drives MAGE cesses has enabled precise, multiplex editing of microbial genomes and DIvERGE (4, 32, 33). This method was first described in and the construction of billions of customized genetic variants in a E. coli, and is most commonly promoted by the expression of bet single day. The techniques that enable this, multiplex automated ge- (here referred to as Redβ) from the Red operon of Escherichia nome engineering (MAGE) and directed evolution with random ge- phage λ (5, 6, 34). Redβ is an ssDNA-annealing protein (SSAP) nomic mutations (DIvERGE), are however, currently limited to a whose role in recombineering is to anneal ssDNA to compli- handful of microorganisms for which single-stranded DNA-annealing mentary genomic DNA at the replication fork. Although im- proteins (SSAPs) that promote efficient recombineering have been provements to recombineering efficiency have been made (7–9), identified. Thus, to enable genome-scale engineering in new hosts, the core protein machinery has remained constant, with Redβ efficient SSAPs must first be found. -

The Efficiency for Recombineering Is Dependent on the Source of The
bioRxiv preprint doi: https://doi.org/10.1101/745448; this version posted August 24, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 The efficiency for recombineering is dependent on the source of the 2 phage recombinase function unit 3 Yizhao Chang, a Qian Wang, b Tianyuan Su, a Qingsheng Qia,c # 4 a State Key Laboratory of Microbial Technology, Shandong University, No. 72 Binhai Road, 5 Qingdao 266237, P.R. China 6 7 b National Glycoengineering Center, Shandong University, Jinan, Shandong, China 8 c CAS Key Lab of Biobased Materials, Qingdao Institute of Bioenergy and Bioprocess 9 Technology, Chinese Academy of Sciences, Qingdao, Shandong, China 10 11 Running Head: Features affecting recombineering efficiency 12 13 # Address correspondence to Qingsheng Qi, [email protected]. 14 15 Word counts for abstract: 183 16 Word counts for text: 3621 17 18 19 20 bioRxiv preprint doi: https://doi.org/10.1101/745448; this version posted August 24, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 21 Abstract 22 Phage recombinase function units (PRFUs) such as lambda-Red or Rac RecET have been proven 23 to be powerful genetic tools in the recombineering of Escherichia coli. -

Manipulating the Mouse Genome Using Recombineering
e in G net ts ic n E e n Biswas and Sharan, Adv Genet Eng 2013, 2:2 g m i e n c e e n DOI: 10.4172/2169-0111.1000108 r a i v n d g A Advancements in Genetic Engineering ISSN: 2169-0111 Review Article Open Access Manipulating the Mouse Genome Using Recombineering Kajal Biswas and Shyam K Sharan* Mouse Cancer Genetics Program, Center for Cancer Research, National Cancer Institute at Frederick, Frederick, Maryland 21702, USA Abstract Genetically engineered mouse models are indispensable for understanding the biological function of genes, understanding the genetic basis of human diseases, and for preclinical testing of novel therapies. Generation of such mouse models has been possible because of our ability to manipulate the mouse genome. Recombineering is a highly efficient, recombination-based method of genetic engineering that has revolutionized our ability to generate mouse models. Since recombineering technology is not dependent on the availability of restriction enzyme recognition sites, it allows us to modify the genome with great precision. It requires homology arms as short as 40 bases for recombination, which makes it relatively easy to generate targeting constructs to insert, change, or delete either a single nucleotide or a DNA fragment several kb in size; insert selectable markers or reporter genes; or add epitope tags to any gene of interest. In this review, we focus on the development of recombineering technology and its application to the generation of genetically engineered mouse models. High-throughput generation of gene targeting vectors, used to construct knockout alleles in mouse embryonic stem cells, is now feasible because of this technology. -

Identification and Analysis of Recombineering Functions from Gram-Negative and Gram-Positive Bacteria and Their Phages
Identification and analysis of recombineering functions from Gram-negative and Gram-positive bacteria and their phages Simanti Datta, Nina Costantino, Xiaomei Zhou, and Donald L. Court† Gene Regulation and Chromosome Biology Laboratory, Center for Cancer Research, National Cancer Institute at Frederick, Frederick, MD 21702 Edited by Sankar Adhya, National Institutes of Health, Bethesda, MD, and approved December 12, 2007 (received for review September 25, 2007) We report the identification and functional analysis of nine genes but linear DNA recombination studies with RecET have used from Gram-positive and Gram-negative bacteria and their phages Gam to inhibit nucleases (4). that are similar to lambda () bet or Escherichia coli recT. Beta and For recombineering, either a dsDNA PCR product (1, 4, RecT are single-strand DNA annealing proteins, referred to here as 15–17) or an oligonucleotide (oligo) (18–21) carrying short recombinases. Each of the nine other genes when expressed in E. (Ϸ50-bp) segments homologous to the target sequences can be coli carries out oligonucleotide-mediated recombination. To our used. These linear DNA substrates are precisely recombined in knowledge, this is the first study showing single-strand recombi- vivo by the phage proteins to target DNA on any replicon. When nase activity from diverse bacteria. Similar to bet and recT, most of linear dsDNA is used for recombination, both the exonuclease these other recombinases were found to be associated with pu- and single-strand annealing protein are required. For optimal tative exonuclease genes. Beta and RecT in conjunction with their recombination, RecBCD should be inactivated, either by muta- cognate exonucleases carry out recombination of linear double- tion or by Gam (1, 15, 22). -

Recombineering Using the Modified DH10B Strain DY380
Recombineering protocol #1 Recombineering using the modified DH10B strain DY380 by Søren Warming, Ph.D. Overview 1. Design primers with 50 bp homology suited for either targeting (insertion of a selectable marker into a specific site) or gap repair (retrieving a piece of DNA into a linear piece of vector backbone). 2. Run PCR and digest residual plasmid template away with DpnI (1-2 ml in a standard 25 ml PCR reaction, @37°C for 1 h). DpnI works fine in PCR buffers. Gel purify the PCR product and elute in ddH2O. If concentration is low, EtOH precipitate and dissolve in small volume of ddH2O. You need 2 x 100-300 ng rather concentrated PCR product per experiment. 3. Alternatively, design primers to amplify “mini-arms” of 250-500 bp length, and clone these arms into an appropiate vector using conventional cloning techniques. The vector is prepared by digestion/linearization and gel purification of the desired band. Background will be higher than with DpnI digested PCR fragments, but the efficiency is very high when the arms are longer. 4. Transform your target plasmid (or BAC) into electrocompetent DY380 cells, see below. Alternatively, co-transform target plasmid and PCR product (see note below). 5. From a single isolated colony, inoculate a 5 ml o/n culture with appropriate selection 6. Perform the heat shock induction, followed by transformation (the recombineering experiment). Transformation (electroporation) of DY380 1. Make a 5 ml o/n culture either from the DY380 freeze stock or from a colony containing a target plasmid. IMPORTANT: Keep the cells @ 32°C or lower. -

Transposon-Mediated BAC Transgenesis in Zebrafish
PROTOCOL Transposon-mediated BAC transgenesis in zebrafish Maximiliano L Suster1,2, Gembu Abe1, Anders Schouw2 & Koichi Kawakami1,3 1Division of Molecular and Developmental Biology, National Institute of Genetics, Mishima, Shizuoka, Japan. 2Sars International Center for Marine Molecular Biology, University of Bergen, Bergen, Norway. 3Department of Genetics, Graduate University for Advanced Studies (SOKENDAI), Mishima, Shizuoka, Japan. Correspondence should be addressed to M.L.S. ([email protected]) or K.K. ([email protected]). Published online 1 December 2011; doi:10.1038/nprot.2011.416 Bacterial artificial chromosomes (BACs) are widely used in studies of vertebrate gene regulation and function because they often closely recapitulate the expression patterns of endogenous genes. Here we report a step-by-step protocol for efficient BAC transgenesis in zebrafish using the medaka Tol2 transposon. Using recombineering in Escherichia coli, we introduce the iTol2 cassette in the BAC plasmid backbone, which contains the inverted minimal cis-sequences required for Tol2 transposition, and a reporter gene to replace a target locus in the BAC. Microinjection of the Tol2-BAC and a codon-optimized transposase mRNA into fertilized eggs results in clean integrations in the genome and transmission to the germline at a rate of ~15%. A single person can prepare a dozen constructs within 3 weeks, and obtain transgenic fish within approximately 3–4 months. Our protocol drastically reduces the labor involved in BAC transgenesis and will greatly facilitate biological and biomedical studies in model vertebrates. INTRODUCTION BAC transgenesis has been used extensively in a broad range of appli- BAC concatemers at a single genomic locus; the remaining carry cations in mice, from studies of gene regulation to creating animal between 5 and 48 copies of the BAC in various orientations15. -

MIE Review.Pdf
[15] recombineering 171 [15] Recombineering: In Vivo Genetic Engineering in E. coli, S. enterica, and Beyond By JAMES A. SAWITZKE,LYNN C. THOMASON,NINA COSTANTINO, MIKHAIL BUBUNENKO,SIMANTI DATTA, and DONALD L. COURT Abstract ‘‘Recombineering,’’ in vivo genetic engineering with short DNA homo- logies, is changing how constructs are made. The methods are simple, precise, efficient, rapid, and inexpensive. Complicated genetic constructs that can be difficult or even impossible to make with in vitro genetic engineering can be created in days with recombineering. DNA molecules that are too large to manipulate with classical techniques are amenable to recombineering. This technology utilizes the phage l homologous recom- bination functions, proteins that can efficiently catalyze recombination between short homologies. Recombineering can be accomplished with linear PCR products or even single‐stranded oligos. In this chapter we discuss methods of and ways to use recombineering. Introduction What Is Recombineering? In vivo genetic engineering using the bacteriophage lambda (l) recom- bination proteins and short DNA homologies has been termed ‘‘recombi- neering’’ (recombination‐mediated genetic engineering)(Ellis et al., 2001) and is the subject of this chapter. Genetic engineering has been instrumental in revolutionizing studies in molecular biology for over 30 years since the discovery of restriction enzymes. Escherichia coli has been the standard host used to recover the products of this in vitro genetic engineering. Since the late 1990s, however, new in vivo technologies have emerged that greatly simplify, accelerate, and expand genetic engineering in E. coli, Salmonella enterica, and other organisms. Now, within a week a researcher can modify any nucleotide(s) of choice in almost any manner. -

Oligo Optimization.Pdf
doi:10.1016/j.jmb.2011.01.030 J. Mol. Biol. (2011) 407,45–59 Contents lists available at www.sciencedirect.com Journal of Molecular Biology journal homepage: http://ees.elsevier.com.jmb Probing Cellular Processes with Oligo-Mediated Recombination and Using the Knowledge Gained to Optimize Recombineering☆ James A. Sawitzke1, Nina Costantino1, Xin-tian Li1, Lynn C. Thomason2, Mikhail Bubunenko1,2, Carolyn Court3 and Donald L. Court1⁎ 1Molecular Control and Genetics Section, Gene Regulation and Chromosome Biology Laboratory, Center for Cancer Research, National Cancer Institute at Frederick, Frederick, MD 21702, USA 2Gene Regulation and Chromosome Biology Laboratory, Basic Science Program, SAIC-Frederick, Inc., National Cancer Institute at Frederick, Frederick, MD 21702, USA 3Transcription Control Section, Gene Regulation and Chromosome Biology Laboratory, Center for Cancer Research, National Cancer Institute at Frederick, Frederick, MD 21702, USA Received 3 December 2010; Recombination with single-strand DNA oligonucleotides (oligos) in received in revised form Escherichia coli is an efficient and rapid way to modify replicons in vivo. 12 January 2011; The generation of nucleotide alteration by oligo recombination provides accepted 13 January 2011 novel assays for studying cellular processes. Single-strand exonucleases Available online inhibit oligo recombination, and recombination is increased by mutating all 19 January 2011 four known exonucleases. Increasing oligo concentration or adding nonspecific carrier oligo titrates out the exonucleases. In a model for oligo Edited by R. Ebright recombination, λ Beta protein anneals the oligo to complementary single- strand DNA at the replication fork. Mismatches are created, and the Keywords: methyl-directed mismatch repair (MMR) system acts to eliminate the mismatch repair; mismatches inhibiting recombination.